Intest Res.
2019 Jan;17(1):94-106. 10.5217/ir.2018.00048.
Long-term prognosis of Japanese patients with biologic-naïve Crohn’s disease treated with anti-tumor necrosis factor-α antibodies
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan. kendo@med.tohoku.ac.jp
- 2Division of Gastroenterology and Hepatology, Tohoku Medical and Pharmaceutical University, Sendai, Japan.
- KMID: 2438448
- DOI: http://doi.org/10.5217/ir.2018.00048
Abstract
- BACKGROUND/AIMS
Few reports have described the long-term treatment outcomes of the anti-tumor necrosis factor-α antibody for Japanese Crohn's disease (CD) patients. The aim of this study was to evaluate them and clarify the clinical factors that affect the long-term prognosis of the anti-tumor necrosis factor-α treatments.
METHODS
This was a retrospective, observational, single-center cohort study. Japanese CD patients treated with either infliximab or adalimumab as a first-line therapy were analyzed. The cumulative retention rates of the biologics, relapse-free survival, and surgery-free survival were analyzed using Kaplan-Meier methods. The clinical factors associated with the long-term outcomes were estimated by both the log-rank test and Cox proportional hazard model.
RESULTS
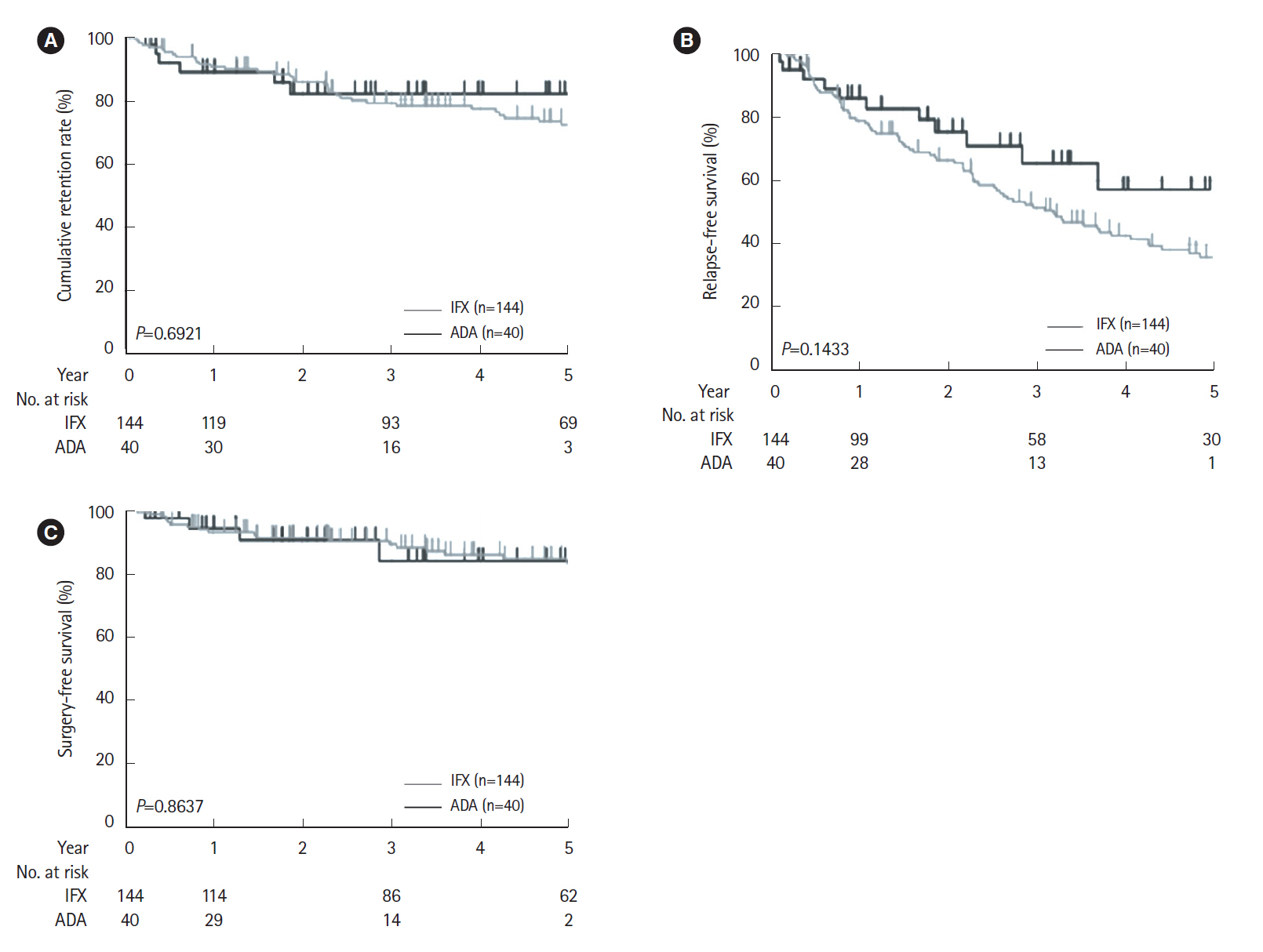
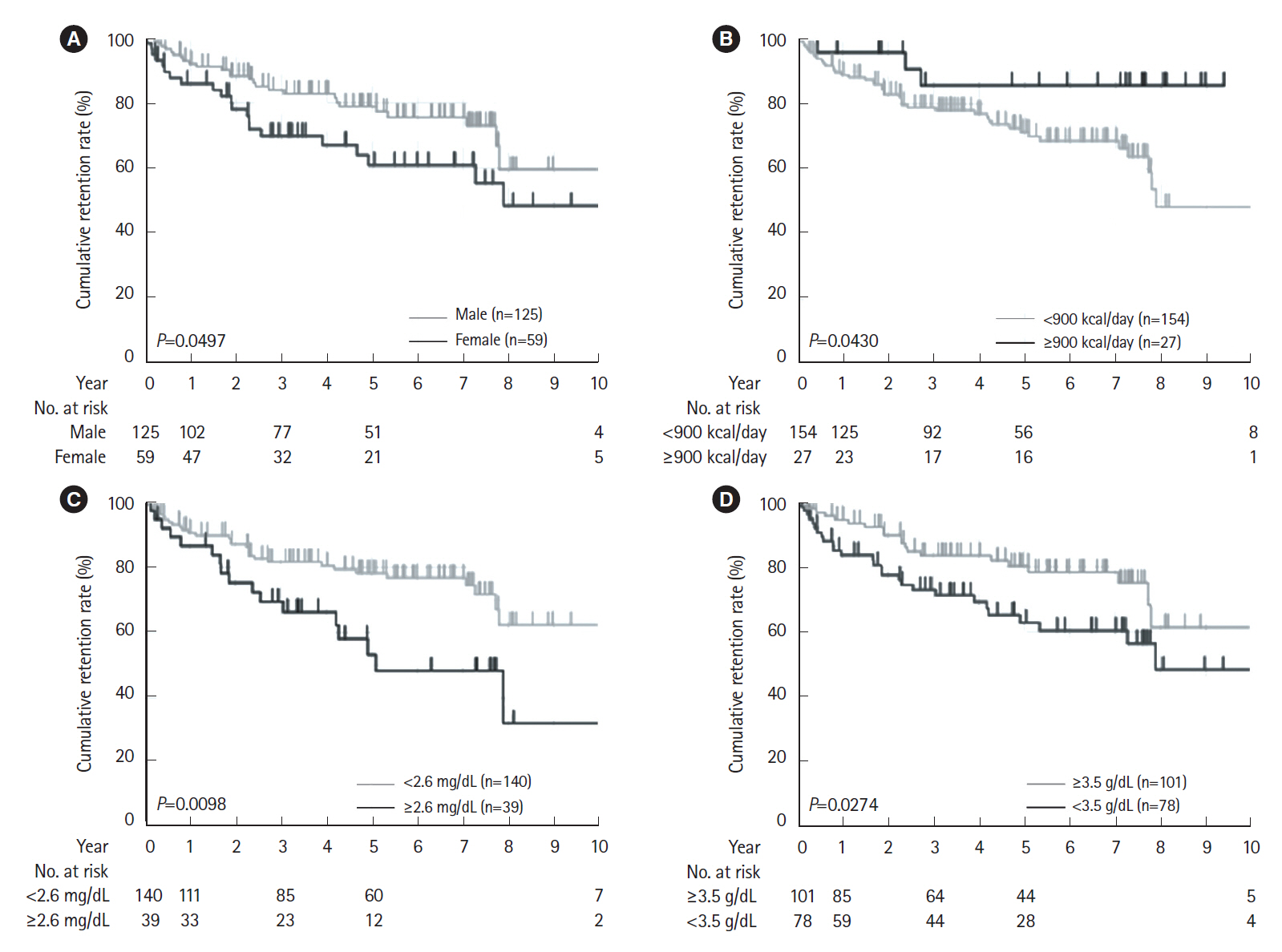
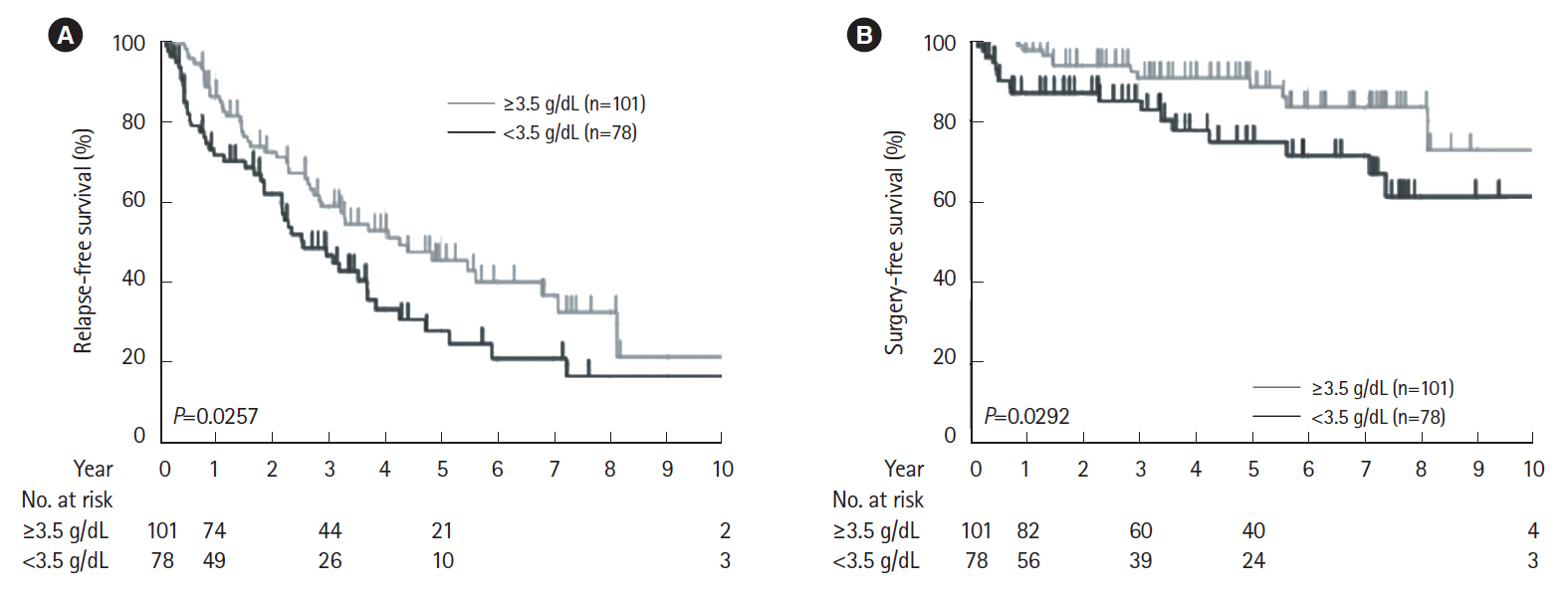
The cumulative retention rate was significantly higher in the group with a concomitant elemental diet of ≥900 kcal/day, baseline C-reactive protein (CRP) levels < 2.6 mg/dL, and baseline serum albumin levels ≥3.5 g/dL, respectively. The baseline serum albumin levels were also associated with both relapse-free and surgery-free survival. The lack of concomitant use of an elemental diet ≥900 kcal/day was identified as the only independent risk factor for the withdrawal of the biologics.
CONCLUSIONS
Baseline CRP levels and serum albumin levels could affect the long-term outcomes in CD patients. Concomitant elemental diet of ≥900 kcal/day could have a positive influence on clinical treatment course.
Keyword
MeSH Terms
-
Adalimumab
Antibodies*
Asian Continental Ancestry Group*
Biological Products
C-Reactive Protein
Cohort Studies
Crohn Disease
Food, Formulated
Humans
Infliximab
Necrosis*
Prognosis*
Proportional Hazards Models
Retrospective Studies
Risk Factors
Serum Albumin
Adalimumab
Antibodies
Biological Products
C-Reactive Protein
Infliximab
Serum Albumin
Figure
Cited by 2 articles
-
Long-term clinical and real-world experience with Crohn’s disease treated with anti-tumor necrosis factor-α antibodies
Haruka Otake, Satohiro Matsumoto, Hirosato Mashima
Intest Res. 2022;20(4):464-474. doi: 10.5217/ir.2021.00139.Natural history of inflammatory bowel disease: a comparison between the East and the West
Eun Mi Song, Suk-Kyun Yang
Intest Res. 2022;20(4):418-430. doi: 10.5217/ir.2021.00104.
Reference
-
1. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012; 142:46–54. e42.
Article2. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012; 380:1590–1605.
Article3. Oriuchi T, Hiwatashi N, Kinouchi Y, et al. Clinical course and long-term prognosis of Japanese patients with Crohn’s disease: predictive factors, rates of operation, and mortality. J Gastroenterol. 2003; 38:942–953.
Article4. Sato Y, Matsui T, Yano Y, et al. Long-term course of Crohn’s disease in Japan: incidence of complications, cumulative rate of initial surgery, and risk factors at diagnosis for initial surgery. J Gastroenterol Hepatol. 2015; 30:1713–1719.
Article5. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.
Article6. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006; 130:323–333.
Article7. Sands BE, Blank MA, Patel K, van Deventer SJ; ACCENT II Study. Long-term treatment of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II Study. Clin Gastroenterol Hepatol. 2004; 2:912–920.
Article8. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.
Article9. Eshuis EJ, Peters CP, van Bodegraven AA, et al. Ten years of infliximab for Crohn’s disease: outcome in 469 patients from 2 tertiary referral centers. Inflamm Bowel Dis. 2013; 19:1622–1630.10. Stein DJ, Ananthakrishnan AN, Issa M, et al. Impact of prior irregular infliximab dosing on performance of long-term infliximab maintenance therapy in Crohn’s disease. Inflamm Bowel Dis. 2010; 16:1173–1179.
Article11. Sprakes MB, Ford AC, Warren L, Greer D, Hamlin J. Efficacy, tolerability, and predictors of response to infliximab therapy for Crohn’s disease: a large single centre experience. J Crohns Colitis. 2012; 6:143–153.
Article12. Bouguen G, Trouilloud I, Siproudhis L, et al. Long-term outcome of non-fistulizing (ulcers, stricture) perianal Crohn’s disease in patients treated with infliximab. Aliment Pharmacol Ther. 2009; 30:749–756.
Article13. Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009; 58:492–500.
Article14. Orlando A, Renna S, Mocciaro F, et al. Six year adalimumab efficacy in steroid-dependent Crohn’s disease patients: a prospective single-center real life study. Dig Liver Dis. 2016; 48:1314–1317.
Article15. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011; 106:674–684.
Article16. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009; 104:760–767.
Article17. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018; 390:2769–2778.
Article18. Vegh Z, Kurti Z, Lakatos PL. Epidemiology of inflammatory bowel diseases from west to east. J Dig Dis. 2017; 18:92–98.
Article19. Fuyuno Y, Yamazaki K, Takahashi A, et al. Genetic characteristics of inflammatory bowel disease in a Japanese population. J Gastroenterol. 2016; 51:672–681.
Article20. Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013; 19:2118–2124.
Article21. Doecke JD, Hartnell F, Bampton P, et al. Infliximab vs. adalimumab in Crohn’s disease: results from 327 patients in an Australian and New Zealand observational cohort study. Aliment Pharmacol Ther. 2017; 45:542–552.
Article22. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012; 91:635–646.
Article23. Rosen MJ, Minar P, Vinks AA. Review article: applying pharmacokinetics to optimise dosing of anti-TNF biologics in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2015; 41:1094–1103.
Article24. Buurman DJ, Maurer JM, Keizer RJ, Kosterink JG, Dijkstra G. Population pharmacokinetics of infliximab in patients with inflammatory bowel disease: potential implications for dosing in clinical practice. Aliment Pharmacol Ther. 2015; 42:529–539.
Article25. Steenholdt C, Bendtzen K, Brynskov J, Ainsworth MA. Optimizing treatment with TNF inhibitors in inflammatory bowel disease by monitoring drug levels and antidrug antibodies. Inflamm Bowel Dis. 2016; 22:1999–2015.
Article26. Fasanmade AA, Adedokun OJ, Blank M, Zhou H, Davis HM. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011; 33:946–964.
Article27. van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. 2013; 9:164–172.
Article28. Takagi S, Utsunomiya K, Kuriyama S, et al. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: a randomized-controlled trial. Aliment Pharmacol Ther. 2006; 24:1333–1340.
Article29. Yamamoto T, Nakahigashi M, Umegae S, Matsumoto K. Prospective clinical trial: enteral nutrition during maintenance infliximab in Crohn’s disease. J Gastroenterol. 2010; 45:24–29.
Article30. Sazuka S, Katsuno T, Nakagawa T, et al. Concomitant use of enteral nutrition therapy is associated with sustained response to infliximab in patients with Crohn’s disease. Eur J Clin Nutr. 2012; 66:1219–1223.
Article31. Hirai F, Ishihara H, Yada S, et al. Effectiveness of concomitant enteral nutrition therapy and infliximab for maintenance treatment of Crohn’s disease in adults. Dig Dis Sci. 2013; 58:1329–1334.
Article32. Kamata N, Oshitani N, Watanabe K, et al. Efficacy of concomitant elemental diet therapy in scheduled infliximab therapy in patients with Crohn’s disease to prevent loss of response. Dig Dis Sci. 2015; 60:1382–1388.
Article33. Nguyen DL, Flores S, Sassi K, Bechtold ML, Nguyen ET, Parekh NK. Optimizing the use of anti-tumor necrosis factor in the management of patients with Crohn’s disease. Ther Adv Chronic Dis. 2015; 6:147–154.
Article34. Sugita N, Watanabe K, Kamata N, et al. Efficacy of a concomitant elemental diet to reduce the loss of response to adalimumab in patients with intractable Crohn’s disease. J Gastroenterol Hepatol. 2018; 33:631–637.
Article35. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010; 362:1383–1395.
Article36. Nakase H, Motoya S, Matsumoto T, et al. Significance of measurement of serum trough level and anti-drug antibody of adalimumab as personalized pharmacokinetics in patients with Crohn’s disease: a subanalysis of the DIAMOND trial. Aliment Pharmacol Ther. 2017; 46:873–882.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of restarting anti-tumor necrosis factor α agents after surgery in patients with Crohn's disease
- Biologic Therapy in Rheumatoid Arthritis
- New drugs for Rheumatoid arthritis
- Comparison of Treatment Guidelines for Ulcerative Colitis: Role of Biologics
- Medical treatment of rheumatoid arthritis (II): current biologic agents and newly developing drugs