Cancer Res Treat.
2019 Jan;51(1):345-356. 10.4143/crt.2018.089.
Longitudinal Assessment of Intravoxel Incoherent Motion Diffusion Weighted Imaging in Evaluating the Radio-sensitivity of Nasopharyngeal Carcinoma Treated with Intensity-Modulated Radiation Therapy
- Affiliations
-
- 1Department of Radiology, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou, China. Yunbinchen@126.com Cheyne09@163.com
- 2Department of Radiation Oncology, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou, China.
- KMID: 2437625
- DOI: http://doi.org/10.4143/crt.2018.089
Abstract
- PURPOSE
Intravoxel incoherent motion diffusion-weighted imaging (IVIM-DWI)was evaluated regarding its ability to preliminarily predict the short-term treatment response of nasopharyngeal carcinoma (NPC) following intensity-modulated radiation therapy.
MATERIALS AND METHODS
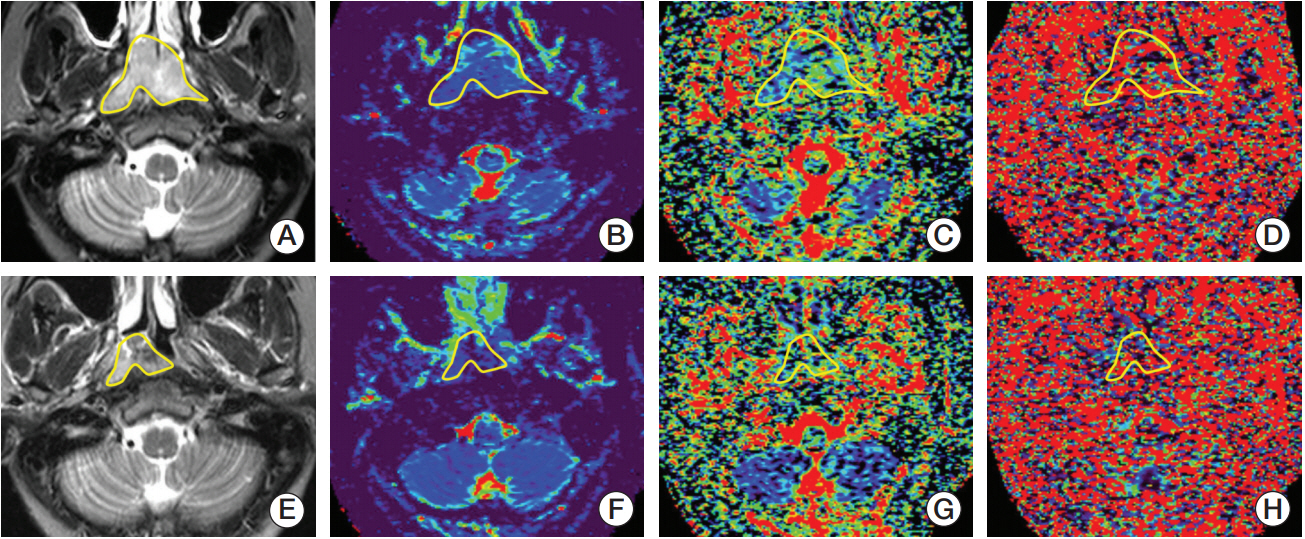
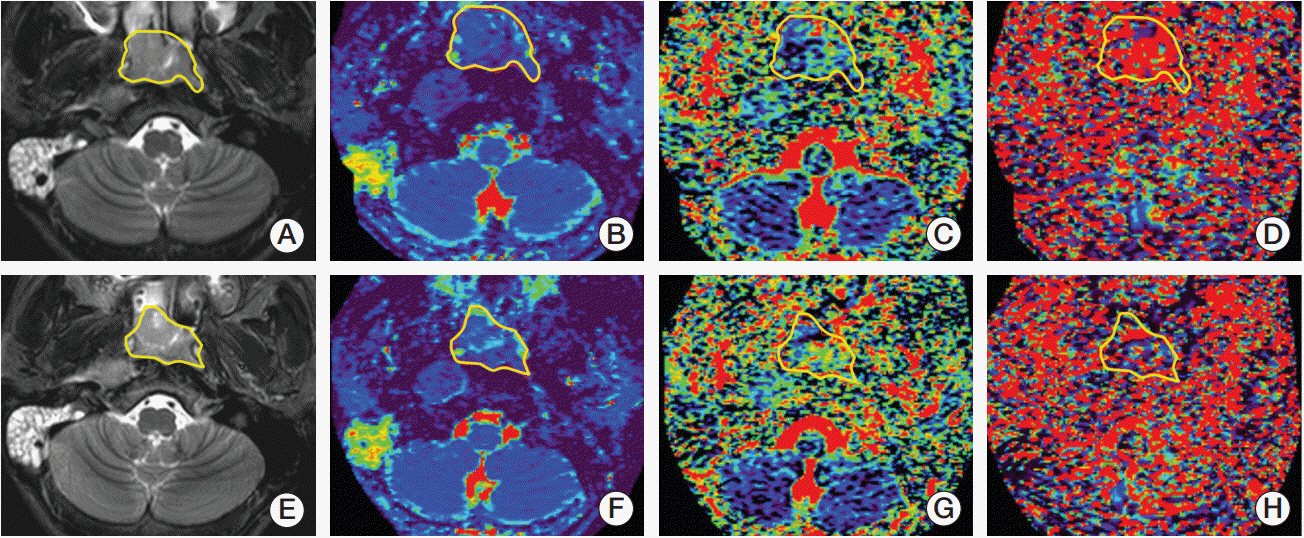
IVIM-DWI with 14 b-factors (0-1,000 sec/mm2) was performed with a 3T MR system on 47 consecutive NPCs before, during (end of the 5th, 10th, 15th, 20th, and 25th fractions), and after fractional radiotherapy. IVIM parametrics (D, f, and D*) were calculated and compared to the baseline and xth fraction. Patients were categorized into responders and non-responders after radiotherapy. IVIM parametrics were also compared between subgroups.
RESULTS
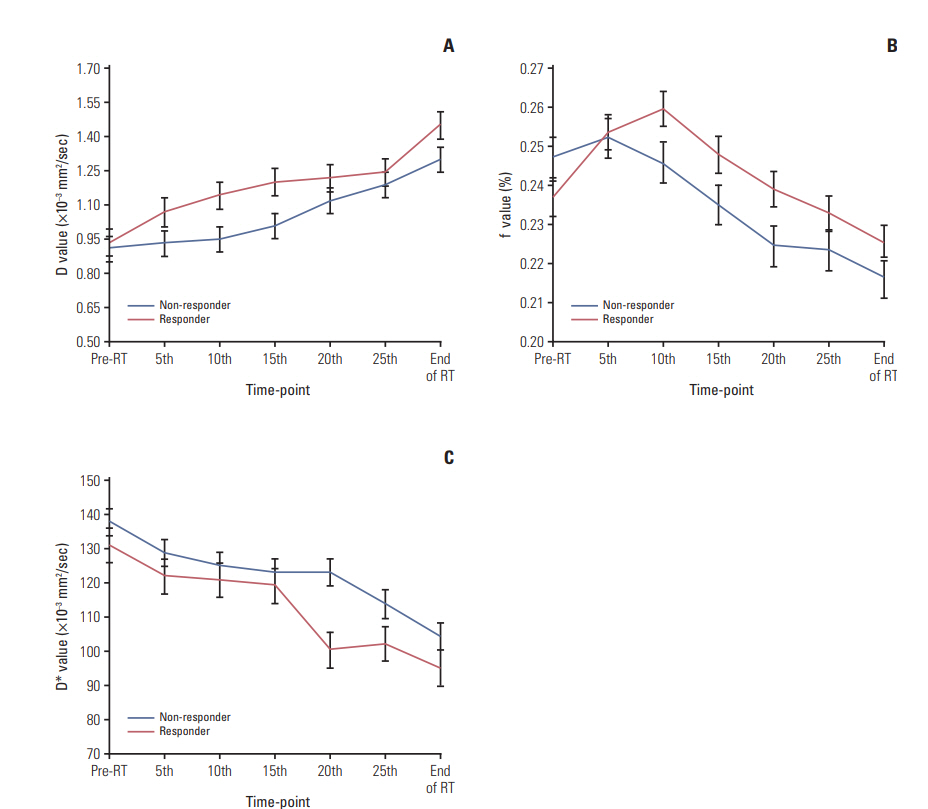
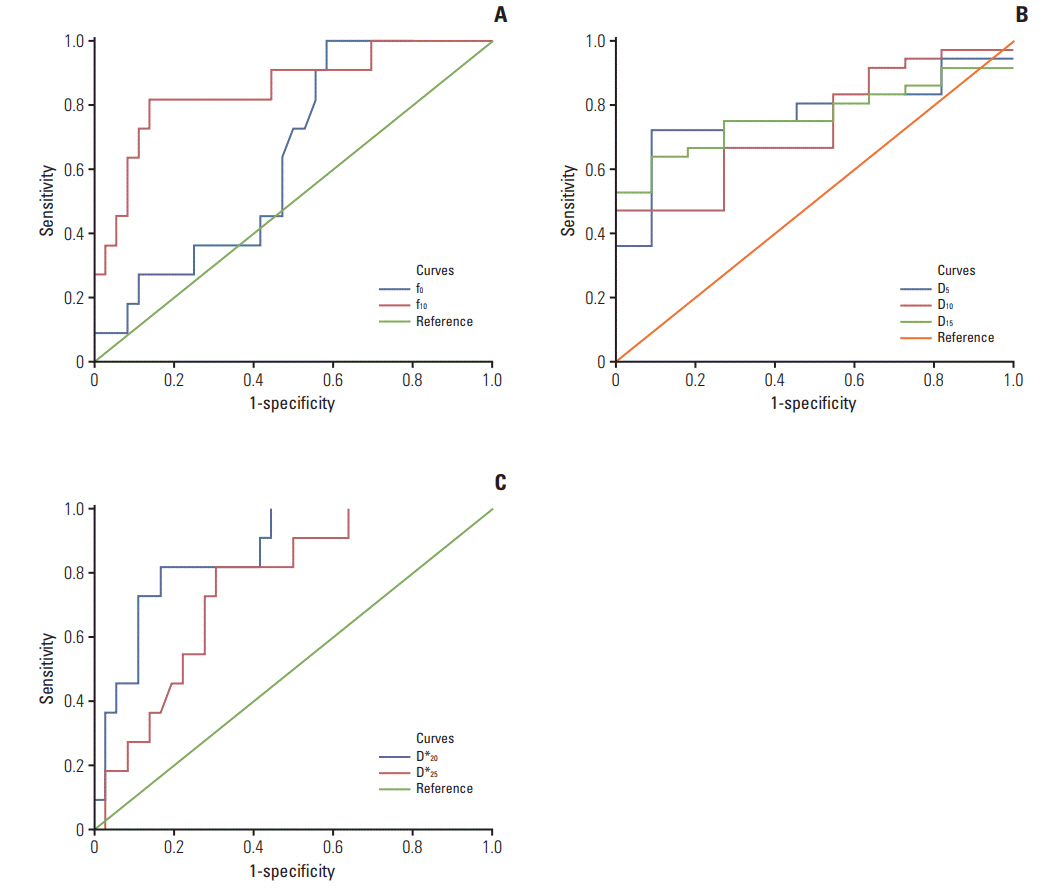
After fractional radiations, the D (except D5 and D at the end of the 5th fraction) after radiations were larger than the baseline D0 (p < 0.05), and the post-radiation D* (except D*5 and D*10) were smaller than D*0 (p < 0.05). f0 was smaller than f5 and f10 (p < 0.001) but larger than fend (p < 0.05). Furthermore, greater D5, D10, D15, and f10 coupled with smaller f0, D*20, and D*25 were observed in responders than non-responders (all p < 0.01). Responders also presented larger ΔD10, Δf10, ΔD*20, and δD*20 than non-responders (p < 0.05). Receiver operating characteristic curve analysis indicated that the D5, D*20, and f10 could better differentiate responders from non-responders.
CONCLUSION
IVIM-DWI could efficiently assess tumor treatment response to fractional radiotherapy and predict the radio-sensitivity for NPCs.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Chua ML, Wee JT, Hui EP, Chan AT. Nasopharyngeal carcinoma. Lancet. 2016; 387:1012–24.
Article2. World Health Organization. GLOBOCAN 2012 v1.0, Estimated cancer incidence, mortality and prevalence worldwide in 2012: IARC Cancer Base No. 11 [Internet]. Lyon: International Agency for Research on Cancer;2012. [cited 2017 Dec 12]. Available from: http://globocan.iarc.fr/Default.aspx.3. Su SF, Han F, Zhao C, Chen CY, Xiao WW, Li JX, et al. Longterm outcomes of early-stage nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy alone. Int J Radiat Oncol Biol Phys. 2012; 82:327–33.
Article4. Lee AW, Ng WT, Chan LL, Hung WM, Chan CC, Sze HC, et al. Evolution of treatment for nasopharyngeal cancer: success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014; 110:377–84.5. Song JH, Wu HG, Keam BS, Hah JH, Ahn YC, Oh D, et al. The role of neoadjuvant chemotherapy in the treatment of nasopharyngeal carcinoma: a multi-institutional retrospective study (KROG 11-06) using propensity score matching analysis. Cancer Res Treat. 2016; 48:917–27.
Article6. Chen YP, Wang ZX, Chen L, Liu X, Tang LL, Mao YP, et al. A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2015; 26:205–11.
Article7. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012; 13:163–71.
Article8. Hong J, Yao Y, Zhang Y, Tang T, Zhang H, Bao D, et al. Value of magnetic resonance diffusion-weighted imaging for the prediction of radiosensitivity in nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2013; 149:707–13.
Article9. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986; 161:401–7.
Article10. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988; 168:497–505.
Article11. Sasaki M, Sumi M, Eida S, Katayama I, Hotokezaka Y, Nakamura T. Simple and reliable determination of intravoxel incoherent motion parameters for the differential diagnosis of head and neck tumors. PLoS One. 2014; 9:e112866.
Article12. Lai V, Li X, Lee VH, Lam KO, Chan Q, Khong PL. Intravoxel incoherent motion MR imaging: comparison of diffusion and perfusion characteristics between nasopharyngeal carcinoma and post-chemoradiation fibrosis. Eur Radiol. 2013; 23:2793–801.
Article13. Lai V, Li X, Lee VH, Lam KO, Fong DY, Huang B, et al. Nasopharyngeal carcinoma: comparison of diffusion and perfusion characteristics between different tumour stages using intravoxel incoherent motion MR imaging. Eur Radiol. 2014; 24:176–83.
Article14. Hauser T, Essig M, Jensen A, Laun FB, Munter M, Maier-Hein KH, et al. Prediction of treatment response in head and neck carcinomas using IVIM-DWI: evaluation of lymph node metastasis. Eur J Radiol. 2014; 83:783–7.
Article15. Cui Y, Zhang C, Li X, Liu H, Yin B, Xu T, et al. Intravoxel incoherent motion diffusion-weighted magnetic resonance imaging for monitoring the early response to ZD6474 from nasopharyngeal carcinoma in nude mouse. Sci Rep. 2015; 5:16389.
Article16. Xiao Y, Pan J, Chen Y, Chen Y, He Z, Zheng X. Intravoxel incoherent motion-magnetic resonance imaging as an early predictor of treatment response to neoadjuvant chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Medicine (Baltimore). 2015; 94:e973.
Article17. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article18. Lin S, Pan J, Han L, Zhang X, Liao X, Lu JJ. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys. 2009; 75:1071–8.
Article19. Khokher S, Qureshi MU, Chaudhry NA. Comparison of WHO and RECIST criteria for evaluation of clinical response to chemotherapy in patients with advanced breast cancer. Asian Pac J Cancer Prev. 2012; 13:3213–8.
Article20. Le Bihan D. Diffusion, confusion and functional MRI. Neuroimage. 2012; 62:1131–6.
Article21. Pan J, Zang L, Zhang Y, Hong J, Yao Y, Zou C, et al. Early changes in apparent diffusion coefficients predict radiosensitivity of human nasopharyngeal carcinoma xenografts. Laryngoscope. 2012; 122:839–43.
Article22. Chawla S, Kim S, Dougherty L, Wang S, Loevner LA, Quon H, et al. Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck. AJR Am J Roentgenol. 2013; 200:35–43.
Article23. Chen Y, Liu X, Zheng D, Xu L, Hong L, Xu Y, et al. Diffusionweighted magnetic resonance imaging for early response assessment of chemoradiotherapy in patients with nasopharyngeal carcinoma. Magn Reson Imaging. 2014; 32:630–7.
Article24. Mardor Y, Roth Y, Ochershvilli A, Spiegelmann R, Tichler T, Daniels D, et al. Pretreatment prediction of brain tumors' response to radiation therapy using high b-value diffusionweighted MRI. Neoplasia. 2004; 6:136–42.
Article25. Lu Y, Jansen JF, Stambuk HE, Gupta G, Lee N, Gonen M, et al. Comparing primary tumors and metastatic nodes in head and neck cancer using intravoxel incoherent motion imaging: a preliminary experience. J Comput Assist Tomogr. 2013; 37:346–52.26. Bisdas S, Braun C, Skardelly M, Schittenhelm J, Teo TH, Thng CH, et al. Correlative assessment of tumor microcirculation using contrast-enhanced perfusion MRI and intravoxel incoherent motion diffusion-weighted MRI: is there a link between them? NMR Biomed. 2014; 27:1184–91.
Article27. King AD, Chow SK, Yu KH, Mo FK, Yeung DK, Yuan J, et al. DCE-MRI for pre-treatment prediction and post-treatment assessment of treatment response in sites of squamous cell carcinoma in the head and neck. PLoS One. 2015; 10:e0144770.
Article28. Larocque MP, Syme A, Allalunis-Turner J, Fallone BG. ADC response to radiation therapy correlates with induced changes in radiosensitivity. Med Phys. 2010; 37:3855–61.
Article29. Koh DM. Science to practice: can intravoxel incoherent motion diffusion-weighted MR imaging be used to assess tumor response to antivascular drugs? Radiology. 2014; 272:307–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RE: Distinguishing between Renal Cell Carcinoma and Fat Poor Angiomyolipoma in Diffusion-Weighted Imaging
- Intravoxel Incoherent Motion MR Imaging in the Head and Neck: Correlation with Dynamic Contrast-Enhanced MR Imaging and Diffusion-Weighted Imaging
- Correlation of the Speed of Enhancement of Hepatic Hemangiomas with Intravoxel Incoherent Motion MR Imaging
- Comparison of Biexponential and Monoexponential Model of Diffusion-Weighted Imaging for Distinguishing between Common Renal Cell Carcinoma and Fat Poor Angiomyolipoma
- B-Value Optimization in the Estimation of Intravoxel Incoherent Motion Parameters in Patients with Cervical Cancer