J Korean Soc Magn Reson Med.
2014 Sep;18(3):208-218. 10.13104/jksmrm.2014.18.3.208.
Correlation of the Speed of Enhancement of Hepatic Hemangiomas with Intravoxel Incoherent Motion MR Imaging
- Affiliations
-
- 1Department of Radiology, Kyung Hee University Hospital at Gangdong, Seoul, Korea. dmy2988@daum.net
- 2Department of Biomedical Engineering, Kyung Hee University, Gyeonggi-do, Korea.
- KMID: 1999920
- DOI: http://doi.org/10.13104/jksmrm.2014.18.3.208
Abstract
- PURPOSE
To evaluate the relationship between the speed of enhancement of hepatic hemangiomas on gadolinium-enhanced MRI and ADC values by using various parameters, including the D, f, D* and ADC(fit) on intravoxel incoherent motion (IVIM) MR Imaging.
MATERIALS AND METHODS
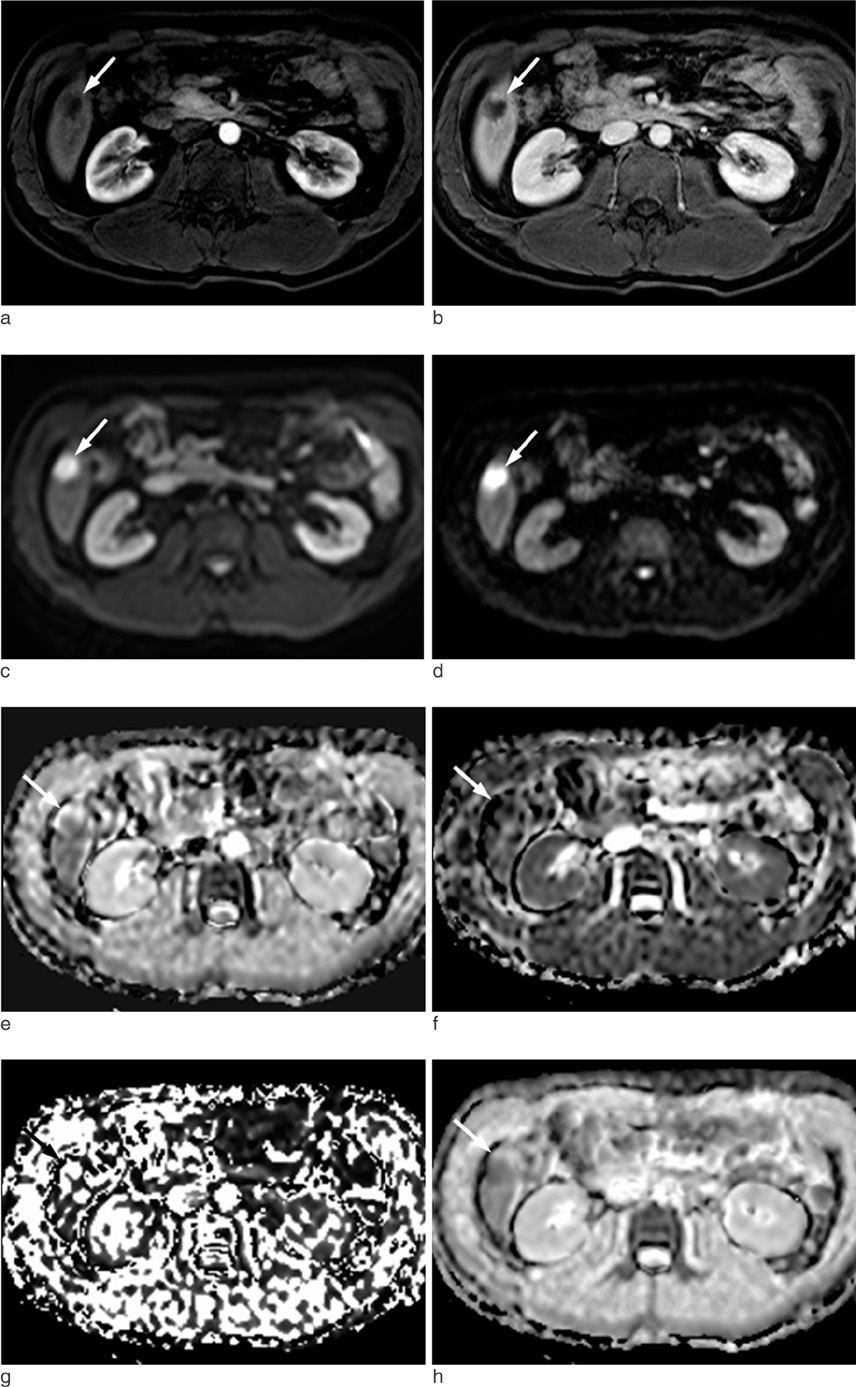
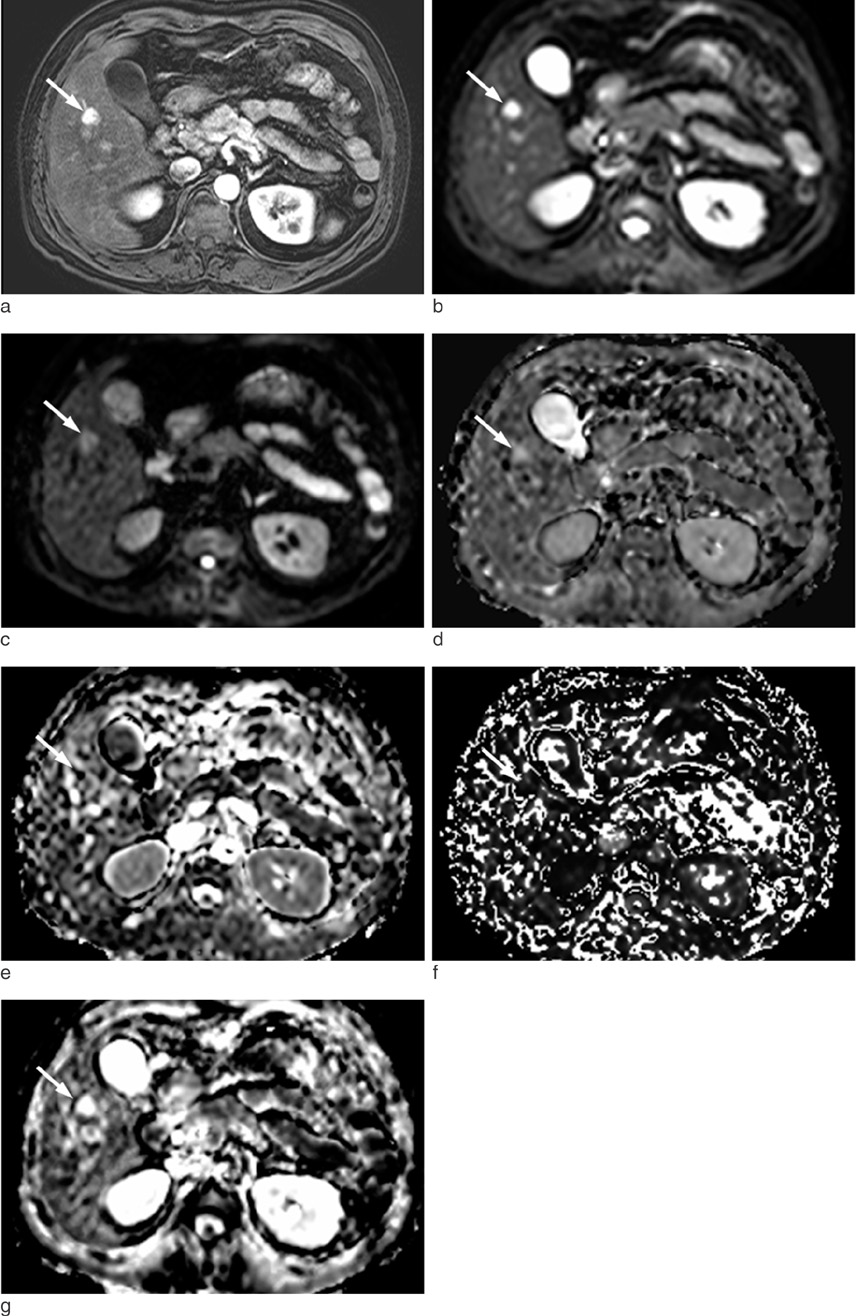
The institutional review board approved this retrospective study. A total of 47 hepatic hemangiomas from 39 patients were included (20 men and 19 women). The hemangiomas were classified into three types according to the enhancement speed of the hepatic hemangiomas on gadolinium-enhanced dynamic T1-weighted images: rapid (Type A), intermediate (Type B), and slow (Type C) enhancement. The D, f, D* and ADC(fit) values were calculated using IVIM MR imaging. The diffusion/perfusion parameters and ADC values were compared among the three types of hemangiomas.
RESULTS
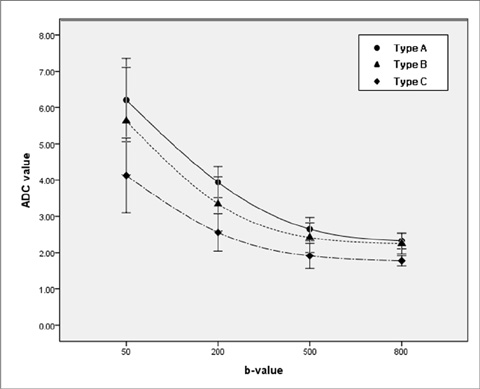
Both the ADC(fit) and D values of type C were significantly lower than those of type A (P = 0.0022, P = 0.0085). However, for the f and D*, there were no significant differences among the three types. On DWI with all b values (50, 200, 500 and 800 sec/mm2), the ADC values of type C were significantly lower than those of the type A (P < 0.012). For b values with 800 sec/mm2, the ADC800 values of the type C hemangiomas were significantly lower than those of type B (P = 0.0021). We found a negative correlation between hepatic hemangioma enhancement type and ADC50 (rho= -0.357, P = 0.014), ADC200 (rho= -0.537, P = 0.0001), ADC500 (rho= -0.614, P = 0.0001), and ADC800(rho= -0.607, P = 0.0001). Therefore, four ADC values of ADC50, ADC200, ADC500, and ADC800 were decreased with decreasing enhancement speed.
CONCLUSION
Hepatic hemangiomas had variable ADCs according to the type of enhancement, and the reduced ADCs in slowly enhancing hemangiomas may be related to the reduced pure molecular diffusion (D).
Keyword
MeSH Terms
Figure
Reference
-
1. Semelka RC, Sofka CM. Hepatic hemangiomas. Magn Reson Imaging Clin N Am. 1997; 5:241–253.2. Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998; 170:397–402.3. Namimoto T, Yamashita Y, Sumi S, Tang Y, Takahashi M. Focal liver masses: characterization with diffusion-weighted echoplanar MR imaging. Radiology. 1997; 204:739–744.4. Taouli B, Vilgrain V, Dumont E, Daire J, Fan B, Menu Y. Evaluation of liver diffusion isotrophy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003; 226:71–78.5. Bruegel M, Holzapfel K, Gaa J, et al. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008; 18:477–485.6. Parikh T, Drew SJ, Lee VS, et al. Focal liver lesion detection and characterization with diffusion weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008; 246:812–822.7. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010; 254:47–66.8. Goshima S, Kanematsu M, Kondo H, et al. Hepatic hemangioma: correlation of enhancement types with diffusion-weighted MR findings and apparent diffusion coefficients. Eur J Radiol. 2009; 70:325–330.9. Vossen JA, Buijs M, Liapi E, Eng J, Bluemke DA, Kamel IR. Receiver operating characteristic analysis of diffusion-weighted magnetic resonance imaging in differentiating hepatic hemangiomas from other hypervascular liver lesions. J Comput Assist Tomogr. 2008; 32:750–756.10. Feuerlein S, Pauls S, Juchems MS, et al. Pitfalls in abdominal diffusion weighted imaging: how predictive is restricted water diffusion for malignancy. AJR Am J Roentgenol. 2009; 193:1070–1076.11. Nam SJ, Park KY, Yu JS, Chung JJ, Kim JH, Kim KW. Hepatic cavernous hemangiomas: relationship between speed of intratumoral enhancement during dynamic MRI and apparent diffusion coefficient on diffusion-weighted imaging. Korean J Radiol. 2012; 13:728–735.12. Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999; 210:617–623.13. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Jeantet ML. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988; 168:497–505.14. Le Bihan D, Turner R, MacFall JR. Effects of intravoxel incoherent motions (IVIM) in steady-state free precession (SSFP) imaging: application to molecular diffusion imaging. Magn Reson Med. 1989; 10:324–337.15. Luciani A, Vignaud A, Cavet M, et al. Liver cirrhosis: intravoxel incoherent motion MR imaging-pilot study. Radiology. 2008; 249:891–899.16. Turner R, Le Bian D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motions. Radiology. 1990; 177:407–414.17. Dixon WT. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging: a modest proposal with tremendous potential. Radiology. 1988; 168:566–567.18. Yoon JH, Lee JM, Yu MH, Kiefer B, Han JK, Choi BI. Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J Magn Reson Imaging. 2014; 39:276–285.19. Ichikawa S, Motosugi U, Ichikawa T, Sano K, Morisaka H, Araki T. Intravoxel incoherent motion imaging of focal hepatic lesions. J Magn Reson Imaging. 2013; 37:1371–1376.20. Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast enhanced MRI alone and in combination: preliminary experience. J Magn Reson Imaging. 2010; 31:589–600.21. Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol. 2011; 196:1351–1361.22. Woo S, Lee JM, Yoon JH, Joo I, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology. 2014; 270:758–767.23. Yu JS, Kim MJ, Kim KW. Intratumoral blood flow in cavernous hemangioma of the liver: radiologic-pathologic correlation. Radiology. 1998; 208:549–550.24. Yamashita Y, Ogata I, Urata J, Takahashi M. Cavernous hemangioma of the liver: pathologic correlation with dynamic CT findings. Radiology. 1997; 203:121–125.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravoxel Incoherent Motion MR Imaging in the Head and Neck: Correlation with Dynamic Contrast-Enhanced MR Imaging and Diffusion-Weighted Imaging
- Hepatic Cavernous Hemangioma in Cirrhotic Liver: Imaging Findings
- Intravoxel Incoherent Motion Magnetic Resonance Imaging for Assessing Parotid Gland Tumors: Correlation and Comparison with Arterial Spin Labeling Imaging
- Dynamic MR Imaging of Hepatic Hemangioma and Hepatocellular: Findings and Differential Diagnosis
- Comparative Study between ZOOMit and Conventional Intravoxel Incoherent Motion MRI for Assessing Parotid Gland Abnormalities in Patients with Early- or Mid-Stage Sjögren’s Syndrome