J Vet Sci.
2019 Jan;20(1):63-71. 10.4142/jvs.2019.20.1.63.
Genome-wide association study of degenerative mitral valve disease in Maltese dogs
- Affiliations
-
- 1Department of Veterinary Laboratory Medicine, College of Veterinary Medicine, Chonnam National University, Gwangju 61186, Korea.
- 2Department of Veterinary Internal Medicine, College of Veterinary Medicine, Konkuk University, Seoul 05030, Korea. parkhee@konkuk.ac.kr
- KMID: 2434779
- DOI: http://doi.org/10.4142/jvs.2019.20.1.63
Abstract
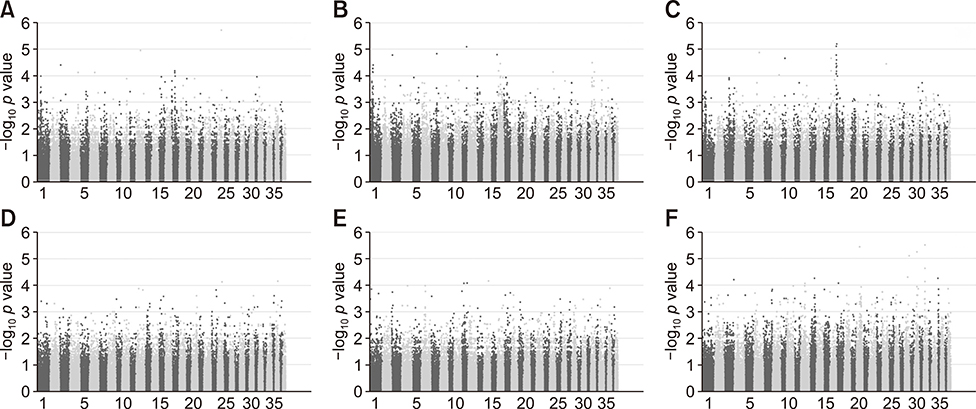
- Genome-wide association study (GWAS) is a powerful tool for identifying the genetic causes of various diseases. This study was conducted to identify genomic variation in Maltese dog genomes associated with degenerative mitral valve disease (DMVD) development and to evaluate the association of each biological condition with DMVD in Maltese dogs. DNA was extracted from blood samples obtained from 48 Maltese dogs (32 with DMVD and 16 controls). Genome-wide single nucleotide polymorphism (SNP) genotyping was performed. The top 30 SNPs from each association of various conditions and genetic variations were mapped to their gene locations. A total of 173,662 loci were successfully genotyped, with an overall genotype completion rate of 99.41%. Quality control analysis excluded 46,610 of these SNPs. Manhattan plots were produced using allelic tests with various candidate clinical conditions. A significant peak of association was observed between mitral valve prolapse (MVP) and SNPs on chromosome 17. The present study revealed significant SNPs in several genes associated with cardiac function, including PDZ2, Armadillo repeat protein detected in velo-cardio-facial syndrome, catenin (cadherin-associated protein) alpha 3, low-density lipoprotein receptor class A domain containing protein 4, and sterile alpha motif domain containing protein 3. To our knowledge, this is the first study of a genetic predisposition to DMVD in Maltese dogs. Although only a limited number of cases were analyzed, these data could be the basis for further research on the genetic predisposition to MVP and DMVD in Maltese dogs.
MeSH Terms
Figure
Reference
-
1. Atkins C, Bonagura J, Ettinger S, Fox P, Gordon S, Haggstrom J, Hamlin R, Keene B, Luis-Fuentes V, Stepien R. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009; 23:1142–1150.
Article2. Aupperle H, Disatian S. Pathology, protein expression and signaling in myxomatous mitral valve degeneration: comparison of dogs and humans. J Vet Cardiol. 2012; 14:59–71.
Article3. Basso C, Fox PR, Meurs KM, Towbin JA, Spier AW, Calabrese F, Maron BJ, Thiene G. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: a new animal model of human disease. Circulation. 2004; 109:1180–1185.
Article4. Bonné S, van Hengel J, van Roy F. Chromosomal mapping of human armadillo genes belonging to the p120ctn/plakophilin subfamily. Genomics. 1998; 51:452–454.
Article5. Borgarelli M, Savarino P, Crosara S, Santilli RA, Chiavegato D, Poggi M, Bellino C, La Rosa G, Zanatta R, Haggstrom J, Tarducci A. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med. 2008; 22:120–128.
Article6. Borgarelli M, Zini E, D'Agnolo G, Tarducci A, Santilli RA, Chiavegato D, Tursi M, Prunotto M, Häggström J. Comparison of primary mitral valve disease in German Shepherd dogs and in small breeds. J Vet Cardiol. 2004; 6:27–34.
Article7. Buchanan JW, Bücheler J. Vertebral scale system to measure canine heart size in radiographs. J Am Vet Med Assoc. 1995; 206:194–199.8. Chen M, Lv Z, Huang L, Zhang W, Lin X, Shi J, Zhang W, Liang R, Jiang S. Triptolide inhibits TGF-β1-induced cell proliferation in rat airway smooth muscle cells by suppressing Smad signaling. Exp Cell Res. 2015; 331:362–368.
Article9. Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation. 2014; 129:2158–2170.
Article10. Freed LA, Acierno JS Jr, Dai D, Leyne M, Marshall JE, Nesta F, Levine RA, Slaugenhaupt SA. A locus for autosomal dominant mitral valve prolapse on chromosome 11p15.4. Am J Hum Genet. 2003; 72:1551–1559.
Article11. French AT, Ogden R, Eland C, Hemani G, Pong-Wong R, Corcoran B, Summers KM. Genome-wide analysis of mitral valve disease in Cavalier King Charles Spaniels. Vet J. 2012; 193:283–286.
Article12. Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000; 1:208–216.
Article13. Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, Birchmeier W. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol. 2004; 167:149–160.
Article14. Hinrichs AL, Larkin EK, Suarez BK. Population stratification and patterns of linkage disequilibrium. Genet Epidemiol. 2009; 33:Suppl 1. S88–S92.
Article15. Hong KW, Shin MS, Ahn YB, Lee HJ, Kim HD. Genomewide association study on chronic periodontitis in Korean population: results from the Yangpyeong health cohort. J Clin Periodontol. 2015; 42:703–710.
Article16. Janssens B, Mohapatra B, Vatta M, Goossens S, Vanpoucke G, Kools P, Montoye T, van Hengel J, Bowles NE, van Roy F, Towbin JA. Assessment of the CTNNA3 gene encoding human αT-catenin regarding its involvement in dilated cardiomyopathy. Hum Genet. 2003; 112:227–236.
Article17. Karlsson EK, Lindblad-Toh K. Leader of the pack: gene mapping in dogs and other model organisms. Nat Rev Genet. 2008; 9:713–725.
Article18. Kraetschmer S, Ludwig K, Meneses F, Nolte I, Simon D. Vertebral heart scale in the beagle dog. J Small Anim Pract. 2008; 49:240–243.
Article19. Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ 3rd, Zody MC, Mauceli E, Xie X, Breen M, Wayne RK, Ostrander EA, Ponting CP, Galibert F, Smith DR, DeJong PJ, Kirkness E, Alvarez P, Biagi T, Brockman W, Butler J, Chin CW, Cook A, Cuff J, Daly MJ, DeCaprio D, Gnerre S, Grabherr M, Kellis M, Kleber M, Bardeleben C, Goodstadt L, Heger A, Hitte C, Kim L, Koepfli KP, Parker HG, Pollinger JP, Searle SM, Sutter NB, Thomas R, Webber C, Baldwin J, Abebe A, Abouelleil A, Aftuck L, Ait-Zahra M, Aldredge T, Allen N, An P, Anderson S, Antoine C, Arachchi H, Aslam A, Ayotte L, Bachantsang P, Barry A, Bayul T, Benamara M, Berlin A, Bessette D, Blitshteyn B, Bloom T, Blye J, Boguslavskiy L, Bonnet C, Boukhgalter B, Brown A, Cahill P, Calixte N, Camarata J, Cheshatsang Y, Chu J, Citroen M, Collymore A, Cooke P, Dawoe T, Daza R, Decktor K, DeGray S, Dhargay N, Dooley K, Dooley K, Dorje P, Dorjee K, Dorris L, Duffey N, Dupes A, Egbiremolen O, Elong R, Falk J, Farina A, Faro S, Ferguson D, Ferreira P, Fisher S, FitzGerald M, Foley K, Foley C, Franke A, Friedrich D, Gage D, Garber M, Gearin G, Giannoukos G, Goode T, Goyette A, Graham J, Grandbois E, Gyaltsen K, Hafez N, Hagopian D, Hagos B, Hall J, Healy C, Hegarty R, Honan T, Horn A, Houde N, Hughes L, Hunnicutt L, Husby M, Jester B, Jones C, Kamat A, Kanga B, Kells C, Khazanovich D, Kieu AC, Kisner P, Kumar M, Lance K, Landers T, Lara M, Lee W, Leger JP, Lennon N, Leuper L, LeVine S, Liu J, Liu X, Lokyitsang Y, Lokyitsang T, Lui A, Macdonald J, Major J, Marabella R, Maru K, Matthews C, McDonough S, Mehta T, Meldrim J, Melnikov A, Meneus L, Mihalev A, Mihova T, Miller K, Mittelman R, Mlenga V, Mulrain L, Munson G, Navidi A, Naylor J, Nguyen T, Nguyen N, Nguyen C, Nguyen T, Nicol R, Norbu N, Norbu C, Novod N, Nyima T, Olandt P, O'Neill B, O'Neill K, Osman S, Oyono L, Patti C, Perrin D, Phunkhang P, Pierre F, Priest M, Rachupka A, Raghuraman S, Rameau R, Ray V, Raymond C, Rege F, Rise C, Rogers J, Rogov P, Sahalie J, Settipalli S, Sharpe T, Shea T, Sheehan M, Sherpa N, Shi J, Shih D, Sloan J, Smith C, Sparrow T, Stalker J, Stange-Thomann N, Stavropoulos S, Stone C, Stone S, Sykes S, Tchuinga P, Tenzing P, Tesfaye S, Thoulutsang D, Thoulutsang Y, Topham K, Topping I, Tsamla T, Vassiliev H, Venkataraman V, Vo A, Wangchuk T, Wangdi T, Weiand M, Wilkinson J, Wilson A, Yadav S, Yang S, Yang X, Young G, Yu Q, Zainoun J, Zembek L, Zimmer A, Lander ES. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005; 438:803–819.
Article20. Madsen MB, Olsen LH, Häggström J, Höglund K, Ljungvall I, Falk T, Wess G, Stephenson H, Dukes-McEwan J, Chetboul V, Gouni V, Proschowsky HF, Cirera S, Karlskov-Mortensen P, Fredholm M. Identification of 2 loci associated with development of myxomatous mitral valve disease in Cavalier King Charles Spaniels. J Hered. 2011; 102:Suppl 1. S62–S67.
Article21. Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010; 363:166–176.
Article22. Nakayama T, Wakao Y, Nemoto H, Uechi M, Kageyama T, Muto M, Takahashi M. Mitral valve protrusion assessed by use of B-mode echocardiography in dogs with mitral regurgitation. Am J Vet Res. 1996; 57:791–797.23. Nesta F, Leyne M, Yosefy C, Simpson C, Dai D, Marshall JE, Hung J, Slaugenhaupt SA, Levine RA. New locus for autosomal dominant mitral valve prolapse on chromosome 13: clinical insights from genetic studies. Circulation. 2005; 112:2022–2030.
Article24. Olsen LH, Fredholm M, Pedersen HD. Epidemiology and inheritance of mitral valve prolapse in Dachshunds. J Vet Intern Med. 1999; 13:448–456.
Article25. Ostrander EA, Wayne RK. The canine genome. Genome Res. 2005; 15:1706–1716.
Article26. Parker HG, Kilroy-Glynn P. Myxomatous mitral valve disease in dogs: does size matter? J Vet Cardiol. 2012; 14:19–29.
Article27. Parker HG, Kim LV, Sutter NB, Carlson S, Lorentzen TD, Malek TB, Johnson GS, DeFrance HB, Ostrander EA, Kruglyak L. Genetic structure of the purebred domestic dog. Science. 2004; 304:1160–1164.
Article28. Pearson TA, Manolio TA. How to interpret a genome-wide association study. JAMA. 2008; 299:1335–1344.
Article29. Pedersen HD, Kristensen BO, Lorentzen KA, Koch J, Jensen AL, Flagstad A. Mitral valve prolapse in 3-year-old healthy Cavalier King Charles Spaniels. An echocardiographic study. Can J Vet Res. 1995; 59:294–298.30. Raggi P, Callister TQ, Lippolis NJ, Russo DJ. Is mitral valve prolapse due to cardiac entrapment in the chest Cavity? A CT view. Chest. 2000; 117:636–642.
Article31. Stern JA, Hsue W, Song KH, Ontiveros ES, Luis Fuentes V, Stepien RL. Severity of mitral valve degeneration is associated with chromosome 15 loci in whippet dogs. PLoS One. 2015; 10:e0141234.
Article32. Sutter NB, Eberle MA, Parker HG, Pullar BJ, Kirkness EF, Kruglyak L, Ostrander EA. Extensive and breed-specific linkage disequilibrium in Canis familiaris. Genome Res. 2004; 14:2388–2396.33. Terzo E, Di Marcello M, McAllister H, Glazier B, Lo Coco D, Locatelli C, Palermo V, Brambilla PG. Echocardiographic assessment of 537 dogs with mitral valve prolapse and leaflet involvement. Vet Radiol Ultrasound. 2009; 50:416–422.
Article34. Thomas WP, Gaber CE, Jacobs GJ, Kaplan PM, Lombard CW, Moise NS, Moses BL. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. J Vet Intern Med. 1993; 7:247–252.
Article35. Tsai KL, Noorai RE, Starr-Moss AN, Quignon P, Rinz CJ, Ostrander EA, Steiner JM, Murphy KE, Clark LA. Genome-wide association studies for multiple diseases of the German Shepherd Dog. Mamm Genome. 2012; 23:203–211.
Article36. Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet. 2007; 8:749–761.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Familial mitral valve prolapse in a Maltese dog family
- Polymorphism in the serotonin transporter protein gene in Maltese dogs with degenerative mitral valve disease
- Correlation of red cell distribution width and left atrial enlargement in Maltese dogs with myxomatous mitral valve disease in Republic of Korea
- Serum serotonin concentration in small breed dogs with degenerative mitral valve disease
- Echocardiographic parameters and indices in 23 healthy Maltese dogs