Ann Lab Med.
2018 Sep;38(5):440-445. 10.3343/alm.2018.38.5.440.
Evaluation of the Luminex ARIES HSV 1&2 Assay and Comparison with the FTD Neuro 9 and In-house Real-Time PCR Assays for Detecting Herpes Simplex Viruses
- Affiliations
-
- 1Department of Laboratory Medicine, National University Hospital, Singapore. chun_kiat_lee@nuhs.edu.sg
- 2Department of Medicine, National University Health System, Singapore.
- 3Biomedical Institute for Global Health Research and Technology, National University of Singapore, Singapore.
- KMID: 2434733
- DOI: http://doi.org/10.3343/alm.2018.38.5.440
Abstract
- BACKGROUND
Human herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) are responsible for a plethora of human diseases, of which cutaneous and mucocutaneous infections are the most prevalent. In its most severe form, HSV infection can cause meningitis/encephalitis. We compared the Luminex ARIES HSV 1&2 assay (Luminex Corp., Austin, TX, USA), an automated sample-to-result molecular solution, to two non-automated HSV DNA assays.
METHODS
A total of 116 artificial controls were used to determine the analytical performance of the ARIES assay. Controls were prepared by spiking universal transport medium (UTM) and cerebrospinal fluid (CSF) samples from patients who tested negative for HSV by an in-house HSV-1 and -2 DNA assay with reference materials (SeraCare Life Sciences, MA, USA; ZeptoMetrix Corp., MA, USA). Another 117 clinical samples were then used to compare the clinical performance of the ARIES assay with those of an in-house assay and the FTD Neuro 9 assay (Fast Track Diagnostics, Junglinster, Luxembourg).
RESULTS
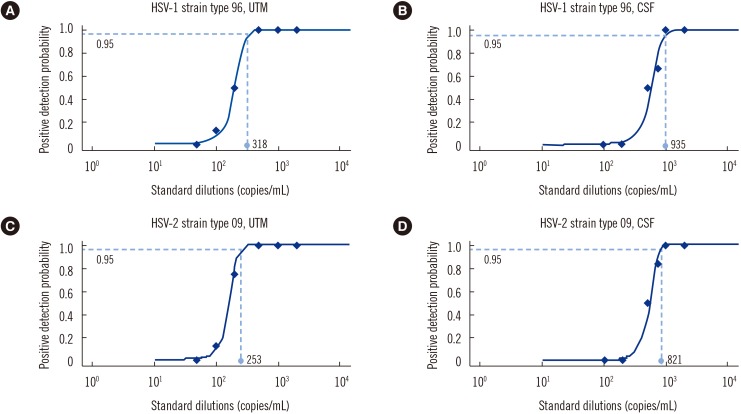
The analytical sensitivity (95% limit of detection) of the ARIES assay was 318 copies/mL (UTM samples) and 935 copies/mL (CSF samples) for HSV-1 strain 96 and 253 copies/mL (UTM samples) and 821 copies/mL (CSF samples) for HSV-2 strain 09. No cross-reactivity was observed in samples spiked with 14 non-HSV microorganisms. Compared with the reference result (agreement between the in-house and FTD Neuro 9 results), the ARIES assay had overall concordance rates of 98.2% (111/113) and 100% (113/113) for HSV-1 and HSV-2, respectively.
CONCLUSIONS
The ARIES assay appears to be an excellent alternative for rapid detection and differentiation of HSV in skin and genital infections, meningitis, and encephalitis.
Keyword
MeSH Terms
Figure
Reference
-
1. Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007; 370:2127–2137. PMID: 18156035.2. Kimberlin DW, Rouse DJ. Clinical practice. Genital herpes. N Engl J Med. 2004; 350:1970–1977. PMID: 15128897.3. World Health Organization. Herpes simplex virus. Updated on Jan 2017. http://www.who.int/mediacentre/factsheets/fs400/en/.4. Wald A. Herpes simplex virus type 2 transmission: risk factors and virus shedding. Herpes. 2004; 11(Suppl 3):130A–137A.5. Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000; 342:844–850. PMID: 10727588.6. Eskild A, Jeansson S, Stray-Pedersen B, Jenum PA. Herpes simplex virus type-2 infection in pregnancy: no risk of fetal death: results from a nested case-control study within 35,940 women. BJOG. 2002; 109:1030–1035. PMID: 12269678.7. Kimberlin DW, Whitley RJ, Wan W, Powell DA, Storch G, Ahmed A, et al. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med. 2011; 365:1284–1292. PMID: 21991950.8. Ellerin TB, Walsh SR, Hooper DC. Recurrent meningitis of unknown aetiology. Lancet. 2004; 363:1772. PMID: 15172776.9. Singh A, Preiksaitis J, Ferenczy A, Romanowski B. The laboratory diagnosis of herpes simplex virus infections. Can J Infect Dis Med Microbiol. 2005; 16:92–98. PMID: 18159535.10. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014; 11:83. PMID: 24885431.11. Kowalski RP, Karenchak LM, Shah C, Gordon JS. ELVIS: a new 24-hour culture test for detecting herpes simplex virus from ocular samples. Arch Ophthamol. 2002; 120:960–962.12. Miller NS, Yen-Lieberman B, Poulter MD, Tang YW, Granato PA. Comparative clinical evaluation of the IsoAmp® HSV assay with ELVIS® HSV culture/ID/typing test system for the detection of herpes simplex virus in genital and oral lesions. J Clin Virol. 2012; 54:355–358. PMID: 22613012.13. Tong Y, McCarthy K, Kong H, Lemieux B. Development and comparison of a rapid isothermal nucleic acid amplification test for typing of herpes simplex virus types 1 and 2 on a portable fluorescence detector. J Mol Diag. 2012; 14:569–576.14. Cone RW, Hobson AC, Brown Z, Ashley R, Berry S, Winter C, et al. Frequent detection of genital herpes simplex virus DNA by polymerase chain reaction among pregnant women. JAMA. 1994; 272:792–796. PMID: 8078144.15. Young S, Body B, Moore F, Dunbar S. Multicenter evaluation of the Luminex® ARIES® HSV 1&2 Assay for the detection of herpes simplex virus types 1 and 2 in cutaneous and mucocutaneous lesion specimens. Expert Rev Mol Diagn. 2016; 16:1241–1249. PMID: 27771977.16. Binnicker MJ, Espy MJ, Duresko B, Irish C, Mandrekar J. Automated processing, extraction and detection of herpes simplex virus types 1 and 2: a comparative evaluation of three commercial platforms using clinical specimens. J Clin Virol. 2017; 89:30–33. PMID: 28226272.17. Tang JW, Lin M, Chiu L, Koay ES. Viral loads of herpes simplex virus in clinical samples–a 5-year retrospective analysis. J Med Virol. 2010; 82:1911–1916. PMID: 20872718.18. Almeida SM, Raboni SM, Noqueira MB, Vidal LR. Red blood cells in cerebrospinal fluid as possible inhibitory factor for enterovirus RT-PCR. Arg Neuropsiquiatr. 2016; 74:810–815.19. Ratnamohan VM, Cunningham AL, Rawlinson WD. Removal of inhibitors of CSF-PCR to improve diagnosis of herpesviral encephalitis. J Virol Methods. 1998; 72:59–65. PMID: 9672133.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug Treatment of Herpes Simplex Infection
- Typing of Herpes Simplex Virus Isolated from Different Sites
- Isolation and Identification of Herpes Simplex Virus Type 2 from Patients with Herpes Progenitalis
- Comparison of the Luminex xTAG Respiratory Viral Panel Fast v2 Assay With Anyplex II RV16 Detection Kit and AdvanSure RV Real-Time RT-PCR Assay for the Detection of Respiratory Viruses
- Detection of Herpes Simplex Virus Type-1 DNA by In Situ Polymerase Chain Reaction