Ann Lab Med.
2012 Nov;32(6):392-398.
Correction of Pseudoreticulocytosis in Leukocytosis Samples Using the Sysmex XE-2100 Analyzer Depends on the Type and Number of White Blood Cells
- Affiliations
-
- 1Department of Laboratory Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. hankja@catholic.ac.kr
Abstract
- BACKGROUND
The reticulocyte count is a good marker of erythropoietic activity of the bone marrow. In the mid-1990s, automated flow cytometric analysis replaced microscopy for the quantification of reticulocytes. Leukocytosis cases with an erroneously high reticulocyte count and a high immature reticulocyte fraction (IRF) have been reported. In this study, we analyzed reticulocyte counts in leukocytosis samples, in an effort to identify a correction method.
METHODS
The study comprised of 21 samples from 16 leukocytosis patients. Results of reticulocyte analyses obtained using a XE-2100 hematology analyzer (Sysmex, Japan) were compared with those obtained using the supravital staining technique, which is a reference method. If the samples showed erroneously high reticulocyte counts and IRF, they were reanalyzed after serial dilution with isotonic solution.
RESULTS
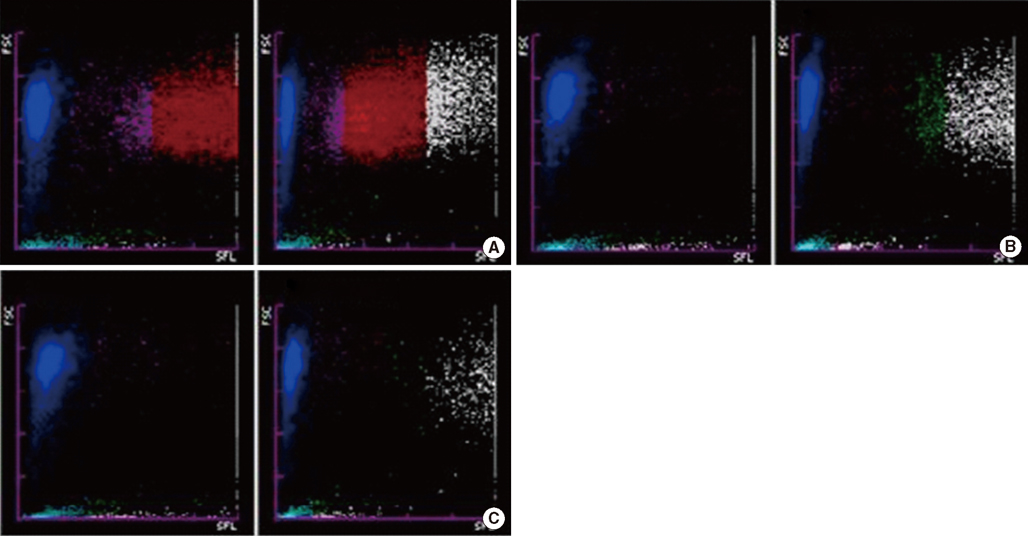
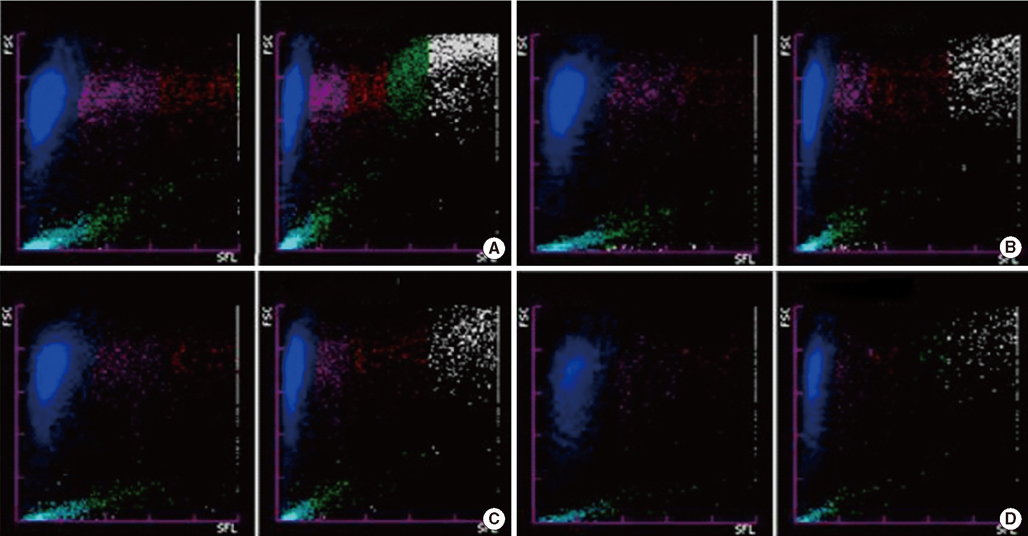
Five samples from 4 patients showed erroneously elevated reticulocyte counts and/or IRF on the XE-2100 analyzer. They displayed abnormal reticulocyte scattergrams, with 4 of 5 cases indicated by a flag. The white blood cell (WBC) fractions overlapped with the reticulocyte regions, especially with the IRF. Diagnoses and blast counts were variable when such errors occurred; WBC counts varied from 218.19x10(9)/L to 725.14x10(9)/L. The errors were corrected by simple dilution with isotonic solution. However, the corrective WBC counts differed according to individual cases.
CONCLUSIONS
When leukocytosis samples exhibit an abnormal reticulocyte scattergram with a flag, or an abnormally high IRF, we recommend the dilution of the sample with isotonic solution to a WBC count of about 100.00x10(9)/L, followed by reanalysis of the reticulocyte count and reticulocyte scattergram.
Keyword
MeSH Terms
Figure
Reference
-
1. Riley RS, Ben-Ezra JM, Goel R, Tidwell A. Reticulocytes and reticulocyte enumeration. J Clin Lab Anal. 2001. 15:267–294.
Article2. Nielsen OJ, Kjaersgaard E, Karle H. Renaissance of the reticulocyte. Ugeskr Laeger. 1994. 156:5673–5675.3. Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem. 2002. 48:1066–1076.
Article4. Deiss A, Kurth D. Circulating reticulocytes in normal adults as determined y the new methylene blue method. Am J Clin Pathol. 1970. 53:481–484.5. Van Petegem M, Cartuyvels R, de Schouwer P, van Duppen V, Goossens W, van Hove L. Comparative evaluation of three flow cytometers for reticulocyte enumeration. Clin Lab Haematol. 1993. 15:103–111.6. Piva E, Brugnara C, Chiandetti L, Plebani M. Automated reticulocyte counting: state of the art and clinical applications in the evaluation of erythropoiesis. Clin Chem Lab Med. 2010. 48:1369–1380.
Article7. Wysocka J, Turowski D. New reticulocyte indices and their utility in hematologic diagnosis. Pol Merkur Lekarski. 2000. 8:498–502.8. Yuh YJ, Kim SR, Han TH. Immature reticulocyte fraction after iron therapy for iron deficiency anemia. Korean J Hematol. 2004. 39:103–108.
Article9. Noronha JF, De Souza CA, Vigorito AC, Aranha FJ, Zulli R, Miranda EC, et al. Immature reticulocytes as an early predictor of engraftment in autologous and allogeneic bone marrow transplantation. Clin Lab Haematol. 2003. 25:47–54.
Article10. Kageoka T. Reticulocyte as indication of the erythroid hematopoiesis: reticulocyte fractions in peripheral blood and bone marrow. Rinsho Byori. 2001. 49:485–489.11. Torres A, Sánchez J, Lakomsky D, Serrano J, Alvarez MA, Martín C, et al. Assessment of hematologic progenitor engraftment by complete reticulocyte maturation parameters after autologous and allogeneic hematopoietic stem cell transplantation. Haematologica. 2001. 86:24–29.12. Briggs C, Harrison P, Grant D, Staves J, MacHin SJ. New quantitative parameters on a recently introduced automated blood cell counter--the XE 2100. Clin Lab Haematol. 2000. 22:345–350.13. Chang CC, Kass L. Clinical significance of immature reticulocyte fraction determined by automated reticulocyte counting. Am J Clin Pathol. 1997. 108:69–73.
Article14. Huh J, Moon H, Chung W. Erroneously elevated immature reticulocyte counts in leukemic patients determined using a Sysmex XE-2100 hematology analyzer. Ann Hematol. 2007. 86:759–762.
Article15. Zandecki M, Genevieve F, Gerard J, Godon A. Spurious counts and spurious results on haematology analysers: a review. Part II: white blood cells, red blood cells, haemoglobin, red cell indices and reticulocytes. Int J Lab Hematol. 2007. 29:21–41.
Article16. Pappas AA, Owens RB, Flick JT. Reticulocyte counting by flow cytometry. A comparison with manual methods. Ann Clin Lab Sci. 1992. 22:125–132.17. Villamor N, Kirsch A, Huhn D, Vives-Corrons JL, Serke S. Interference of blood leucocytes in the measurements of immature red cells (reticulocytes) by two different (semi-) automated flow-cytometry technologies. Clin Lab Haematol. 1996. 18:89–94.
Article18. Laurencet FM, Martinez T, Beris P. Spurious extreme reticulocytosis with an automated reticulocyte analyzer. N Engl J Med. 1997. 337:1922–1923.
Article19. Español I, Pedro C, Remacha AF. Heinz bodies interfere with automated reticulocyte counts. Haematologica. 1999. 84:373–374.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the Mindray BC-6200 Hematology Analyzer; Comparison with Sysmex XE-2100 and Manual Microscopy
- Evaluation of the Automated Hematology Analyzer Sysmex XN-2000 and the Accuracy of Differential Leukocyte Counts Using the Low WBC Mode
- Evaluation of a portable hemoglobin photometer for assessment of intra-operative hemoglobin concentrations
- Three Cases of Pseudoeosinophilia Associated with Malaria Determined in the Sysmex XE-2100 Automated Hematology Analyzer
- Performance Evaluation of the Beckman Coulter DxH 900 Automated Hematology Analyzer