Korean J Ophthalmol.
2019 Feb;33(1):46-53. 10.3341/kjo.2018.0034.
Diabetic Nephropathy in Type 2 Diabetic Retinopathy Requiring Panretinal Photocoagulation
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, The Catholic University of Korea College of Medicine, Seoul, Korea. parkyh@catholic.ac.kr
- 2Catholic Institute for Visual Science, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2434304
- DOI: http://doi.org/10.3341/kjo.2018.0034
Abstract
- PURPOSE
To investigate the risk factors of diabetic nephropathy in patients with diabetic retinopathy requiring panretinal photocoagulation (PRP) and the visual prognosis.
METHODS
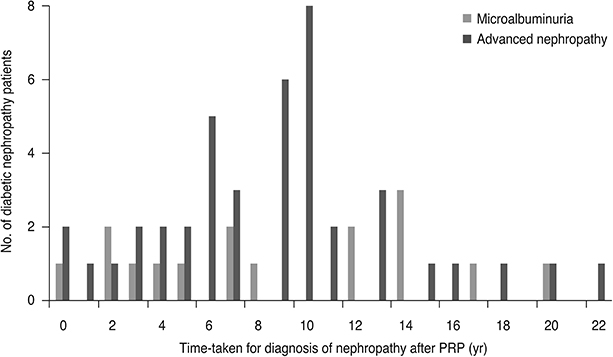
A retrospective review of electronic medical records was conducted at Seoul St. Mary's Hospital, comprising 103 patients with type 2 diabetes mellitus and diabetic retinopathy who underwent PRP from 1996 to 2005. Patients with type 1 diabetes mellitus, non-diabetic renal disease, non-diabetic retinal disease, visually significant ocular disease, high-risk proliferative diabetic retinopathy, and advanced diabetic retinopathy were excluded. The patients were divided into three groups: no nephropathy (group 1, n = 45), microalbuminuria (group 2, n = 16), and advanced nephropathy (group 3, n = 42). Duration of diagnosis of retinopathy and nephropathy, glycosylated hemoglobin, visual acuity, complications, and treatment history were investigated.
RESULTS
The mean glycosylated hemoglobin of group 3 (8.4 ± 1.2) was higher than that of group 1 (7.7 ± 1.0) or group 2 (7.7 ± 1.0) (p = 0.04). Mean interval from PRP to diagnosis of nephropathy was 8.8 ± 6.0 years in group 2 and 8.7 ± 4.9 years in group 3. The significant decrease in visual acuity in group 3 (28 eyes, 35.9%) was significantly higher than that in group 1 (15 eyes, 18.1%, p = 0.01) or group 2 (6 eyes, 20.7%, p = 0.03). Only vitreous hemorrhage showed a significantly higher incidence in groups 2 and 3 than in group 1 (p = 0.02). Multivariate regression analysis revealed that female sex and lower glycosylated hemoglobin were significantly associated with a protective effect on development of nephropathy.
CONCLUSIONS
In the clinical setting, many patients with PRP-requiring diabetic retinopathy develop nephropathy an average of 8 to 9 years after PRP. Male sex and higher glycosylated hemoglobin could be risk factors of nephropathy.
MeSH Terms
Figure
Reference
-
1. Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003; 290:2057–2060.
Article2. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984; 102:520–526.3. Skyler JS. Microvascular complications: retinopathy and nephropathy. Endocrinol Metab Clin North Am. 2001; 30:833–856.4. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981; 88:583–600.5. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991; 98:786–806.6. He F, Xia X, Wu XF, et al. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia. 2013; 56:457–466.
Article7. Man RE, Sasongko MB, Wang JJ, et al. The association of estimated glomerular filtration rate with diabetic retinopathy and macular edema. Invest Ophthalmol Vis Sci. 2015; 56:4810–4816.
Article8. Lee WJ, Sobrin L, Lee MJ, et al. The relationship between diabetic retinopathy and diabetic nephropathy in a population-based study in Korea (KNHANES V-2, 3). Invest Ophthalmol Vis Sci. 2014; 55:6547–6553.
Article9. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991; 98:741–756.10. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997; 20:1183–1197.11. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003; 26:Suppl 1. S5–S20.12. Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991; 98:5 Suppl. 807–822.13. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005; 28:164–176.
Article14. Kohner EM, Stratton IM, Aldington SJ, et al. Microaneurysms in the development of diabetic retinopathy (UKPDS42). UK Prospective Diabetes Study Group. Diabetologia. 1999; 42:1107–1112.15. Palmberg P, Smith M, Waltman S, et al. The natural history of retinopathy in insulin-dependent juvenile-onset diabetes. Ophthalmology. 1981; 88:613–618.
Article16. Andersen AR, Christiansen JS, Andersen JK, et al. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983; 25:496–501.
Article17. Hartnett ME, Key IJ, Loyacano NM, et al. Perceived barriers to diabetic eye care: qualitative study of patients and physicians. Arch Ophthalmol. 2005; 123:387–391.18. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009; 32:1327–1334.19. Wykoff CC. Impact of intravitreal pharmacotherapies including antivascular endothelial growth factor and corticosteroid agents on diabetic retinopathy. Curr Opin Ophthalmol. 2017; 28:213–218.
Article20. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003; 63:225–232.
Article21. Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care. 2004; 27:Suppl 1. S79–S83.
Article22. Liew BS, Perry C, Boulton-Jones JM, et al. Diabetic nephropathy: an observational study on patients attending a joint diabetes renal clinic. QJM. 1997; 90:353–358.
Article23. Lee WJ, Sobrin L, Kang MH, et al. Ischemic diabetic retinopathy as a possible prognostic factor for chronic kidney disease progression. Eye (Lond). 2014; 28:1119–1125.
Article24. Jeng CJ, Hsieh YT, Yang CM, et al. Diabetic retinopathy in patients with diabetic nephropathy: development and progression. PLoS One. 2016; 11:e0161897.
Article25. Takao T, Matsuyama Y, Yanagisawa H, et al. Visit-to-visit variability in systolic blood pressure predicts development and progression of diabetic nephropathy, but not retinopathy, in patients with type 2 diabetes. J Diabetes Complications. 2014; 28:185–190.
Article26. Ciray H, Aksoy AH, Ulu N, et al. Nephropathy, but not angiographically proven retinopathy, is associated with neutrophil to lymphocyte ratio in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2015; 123:267–271.
Article27. Cortes P, Mogensen CE. The diabetic kidney. Totowa: Humana Press;2006. p. 473–498.28. Simo-Servat O, Hernandez C, Simo R. Genetics in diabetic retinopathy: current concepts and new insights. Curr Genomics. 2013; 14:289–299.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macular Thickness and Visual Acuity Before and After Panretinal Photocoagulation in Severe Diabetic Retinopathy
- Retinal Vascular Caliber Changes in Diabetic Retinopathy after Panretinal Photocoagulation and Additive Bevacizumab Injections
- The Changes in Central Macular Thickness after Cataract Surgery in Patients with Diabetic Retinopathy
- Clinical Analysis of Panretinal Photocoagulation for Diabetic Retinopathy
- The Effects of Pa n retinal Photocoagulation on Macular Microcirculation in Diabetic Retinopathy(Short term follow up)