Nutr Res Pract.
2019 Feb;13(1):17-22. 10.4162/nrp.2019.13.1.17.
A 1:1 exercise-to-rest period ratio needed by animals to restore energy sources and replenish anti-oxidative status after exercise
- Affiliations
-
- 1Department of Food and Nutrition, Duksung Women's University, 33 Samyangro 144, Dobong-Gu, Seoul, 01369, South Korea. yunokcho@duksung.ac.kr
- KMID: 2434072
- DOI: http://doi.org/10.4162/nrp.2019.13.1.17
Abstract
- BACKGROUND/OBJECTIVES
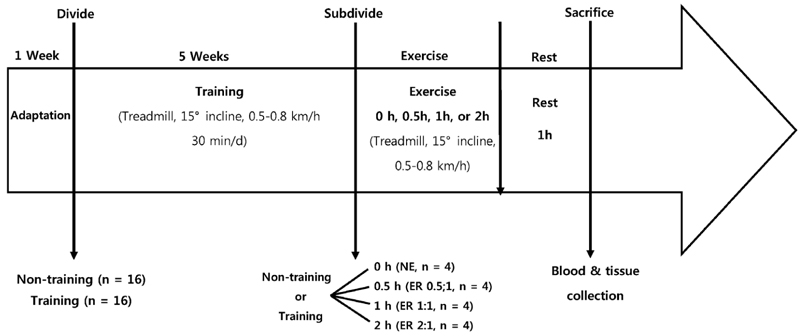
Successful recovery of an animal from exercise is essential, especially prior to the next exercise session. This study was conducted to find an effective exercise-to-rest period ratio for the restoration of energy sources and replenishment of anti-oxidative status in tissue after exercise.
MATERIALS/METHODS
Thirty-two rats were assigned to either non-training or training exercise groups for 5 weeks. After that period, the two groups were subdivided into four smaller groups: non-exercise (NE), exercise 0.5 hour and rest 1 hour (ER0.5:1), exercise 1 hour and rest 1 hour (ER1:1), exercise 2 hours and rest 1 hour (ER2:1).
RESULTS
In the training group animals and compared to the NE group, the levels of plasma glucose after the rest period were significantly high in all ER groups but highest in the ER2:1 group. Similarly, the liver glycogen level was highest in the ER2:1 group. The plasma FFA level reached the highest level in the ER2:1 group but was similarly high in the ER0.5:1 group. Liver TG level was unchanged in the ER2:1 and ER1:1 groups but was significantly high in the ER0.5:1 group. Muscle TG levels were decreased in all three ER groups. Plasma protein levels were significantly high in the ER2:1 and ER0.5:1 groups. In both training animal and non-training animals, the liver protein levels did not change significantly between the NE and ER groups, irrespective of the exercise-to-rest ratio. In the training animal group, muscle protein level was significantly low in the ER2:1 and ER0.5:1 groups. The activity levels of superoxide dismutase and catalase, as well as the malondialdehyde concentration, were not significantly different between NE and ER groups, irrespective of the exercise-to-rest period ratio.
CONCLUSIONS
These results indicate that animals provided with a 0.5:1 to 1:1 exercise-to-rest period ratio can restore their muscle energy sources and recover their anti-oxidative defense system.
Keyword
MeSH Terms
Figure
Reference
-
1. Tsintzas K, Williams C, Boobis L, Symington S, Moorehouse J, Garcia-Roves P, Nicholas C. Effect of carbohydrate feeding during recovery from prolonged running on muscle glycogen metabolism during subsequent exercise. Int J Sports Med. 2003; 24:452–458.
Article2. van Hall G, Shirreffs SM, Calbet JA. Muscle glycogen resynthesis during recovery from cycle exercise: no effect of additional protein ingestion. J Appl Physiol (1985). 2000; 88:1631–1636.
Article3. Williams M, Raven PB, Fogt DL, Ivy JL. Effects of recovery beverages on glycogen restoration and endurance exercise performance. J Strength Cond Res. 2003; 17:12–19.
Article4. Wu CL, Williams C. A low glycemic index meal before exercise improves endurance running capacity in men. Int J Sport Nutr Exerc Metab. 2006; 16:510–527.
Article5. Rowlands DS, Hopkins WG. Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Metabolism. 2002; 51:678–690.
Article6. Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol (1985). 1994; 76:2253–2261.
Article7. Romjin JA, Coyle EF, Sidossis L, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993; 265:E380–E391.8. Barnett C, Carey M, Proietto J, Cerin E, Febbraio MA, Jenkins D. Muscle metabolism during sprint exercise in man: influence of sprint training. J Sci Med Sport. 2004; 7:314–322.
Article9. Stuewe SR, Gwirtz PA, Agarwal N, Mallet RT. Exercise training enhances glycolytic and oxidative enzymes in canine ventricular myocardium. J Mol Cell Cardiol. 2000; 32:903–913.
Article10. Choi EY, Cho YO. Moderate physical training can increase muscle glycogen levels but does not alter protein levels with exercise in rats. Nutr Sci. 2006; 9:112–116.11. Choi EY, Cho YO. The effects of physical training on antioxidative status under exercise-induced oxidative stress. Nutr Res Pract. 2007; 1:14–18.
Article12. Roepstorff C, Vistisen B, Kiens B. Intramuscular triacylglycerol in energy metabolism during exercise in humans. Exerc Sport Sci Rev. 2005; 33:182–188.
Article13. Henderson GC, Alderman BL. Determinants of resting lipid oxidation in response to a prior bout of endurance exercise. J Appl Physiol (1985). 2014; 116:95–103.
Article14. Choi EY, Cho YO. The influence of different durations of aerobic exercise on fuel utilization, lactate level and antioxidant defense system in trained rats. Nutr Res Pract. 2014; 8:27–32.
Article15. Ghanassia E, Brun JF, Mercier J, Raynaud E. Oxidative mechanisms at rest and during exercise. Clin Chim Acta. 2007; 383:1–20.
Article16. O'Reilly KP, Warhol MJ, Fielding RA, Frontera WR, Meredith CN, Evans WJ. Eccentric exercise-induced muscle damage impairs muscle glycogen repletion. J Appl Physiol (1985). 1987; 63:252–256.17. Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006; 86:205–243.
Article18. Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr. 2000; 72:558S–563S.
Article19. Kenney WL, Wilmore JH, Costill DL. Physiology of sport and exercise. Champaign, IL: Human Kinetics;2015. p. 51–72.20. Bülow J, Gjeraa K, Enevoldsen LH, Simonsen L. Lipid mobilization from human abdominal, subcutaneous adipose tissue is independent of sex during steady-state exercise. Clin Physiol Funct Imaging. 2006; 26:205–211.
Article21. Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Mau TL, Luke-Zeitoun M, Brooks GA. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol. 2007; 584:963–981.
Article22. Iwayama K, Kawabuchi R, Park I, Kurihara R, Kobayashi M, Hibi M, Oishi S, Yasunaga K, Ogata H, Nabekura Y, Tokuyama K. Transient energy deficit induced by exercise increases 24-h fat oxidation in young trained men. J Appl Physiol (1985). 2015; 118:80–85.
Article23. Davitt PM, Arent SM, Tuazon MA, Golem DL, Henderson GC. Postprandial triglyceride and free fatty acid metabolism in obese women after either endurance or resistance exercise. J Appl Physiol (1985). 2013; 114:1743–1754.
Article24. Watt MJ, Heigenhauser GJ, Spriet LL. Intramuscular triacylglycerol utilization in human skeletal muscle during exercise: is there a controversy? J Appl Physiol (1985). 2002; 93:1185–1195.
Article25. van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol. 2003; 553:611–625.
Article26. Jensen MD. Fate of fatty acids at rest and during exercise: regulatory mechanisms. Acta Physiol Scand. 2003; 178:385–390.
Article27. Koopman R, Manders RJ, Jonkers RA, Hul GB, Kuipers H, van Loon LJ. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Eur J Appl Physiol. 2006; 96:525–534.
Article28. Lira FS, Koyama CH, Yamashita AS, Rosa JC, Zanchi NE, Batista ML Jr, Seelaender MC. Chronic exercise decreases cytokine production in healthy rat skeletal muscle. Cell Biochem Funct. 2009; 27:458–461.
Article29. Campbell PT, Gross MD, Potter JD, Schmitz KH, Duggan C, McTiernan A, Ulrich CM. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Med Sci Sports Exerc. 2010; 42:1448–1453.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of physical training on antioxidative status under exercise-induced oxidative stress
- Effect of Short Termed Fasting on the Usage Patterns of Metabolic Energy Sources during Exercise in Man
- How Does Obesity and Physical Activity Affect Aging?: Focused on Telomere as a Biomarker of Aging
- Effect of vitamin B6 deficiency on antioxidative status in rats with exercise-induced oxidative stress
- Effects of Resveratrol Supplementation on Oxidative Damage and Lipid Peroxidation Induced by Strenuous Exercise in Rats