J Cardiovasc Imaging.
2019 Jan;27(1):50-63. 10.4250/jcvi.2019.27.e4.
Differential Transcriptome Profile and Exercise Capacity in Cardiac Remodeling by Pressure Overload versus Volume Overload
- Affiliations
-
- 1Department of Internal Medicine, Cardiovascular Center, Sejong General Hospital, Incheon, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea. kimdamas@snu.ac.kr
- KMID: 2433765
- DOI: http://doi.org/10.4250/jcvi.2019.27.e4
Abstract
- BACKGROUND
We compared the gene expression profiles in the hypertrophied myocardium of rats subjected to pressure overload (PO) and volume overload (VO) using DNA chip technology, and compared the effects on exercise capacity with a treadmill test.
METHODS
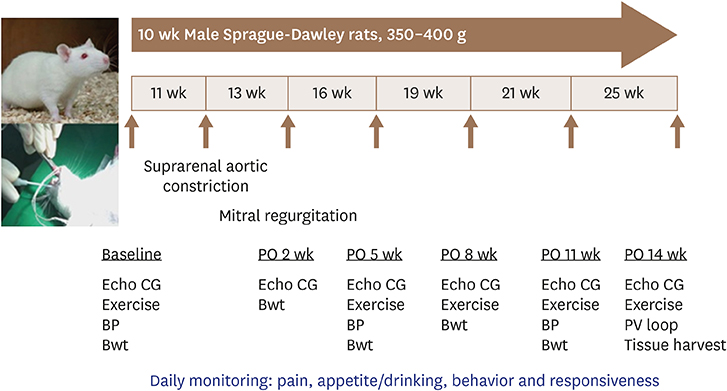
Constriction of the abdominal aorta or mitral regurgitation induced by a hole in the mitral leaflet were used to induce PO (n = 19), VO (n = 16) or PO + VO (n = 20) in rats. Serial echocardiographic studies and exercise were performed at 2-week intervals, and invasive hemodynamic examination by a pressure-volume catheter system was performed 12 weeks after the procedure. The gene expression profiles of the left ventricle (LV) 12 weeks after the procedure were analyzed by DNA chip technology.
RESULTS
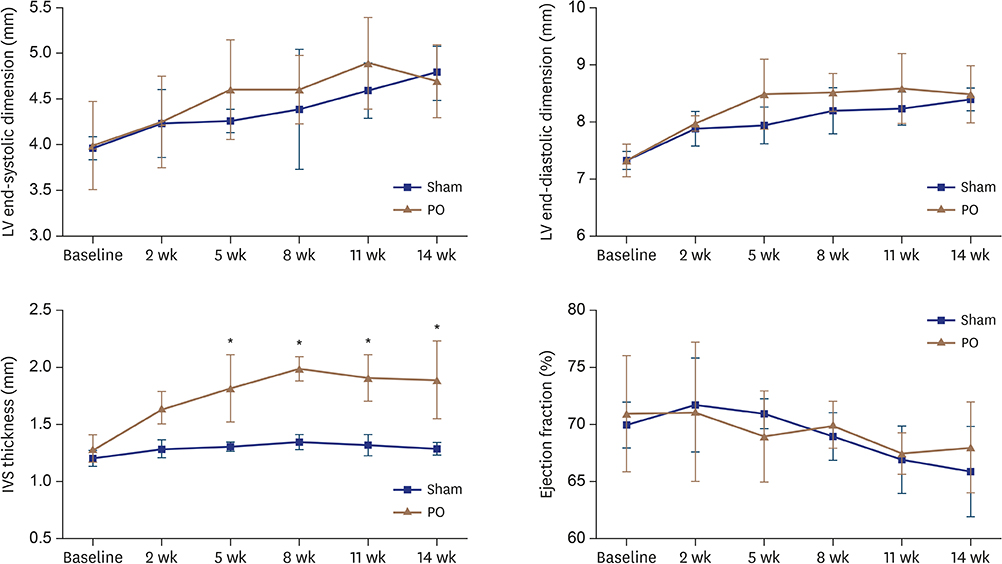
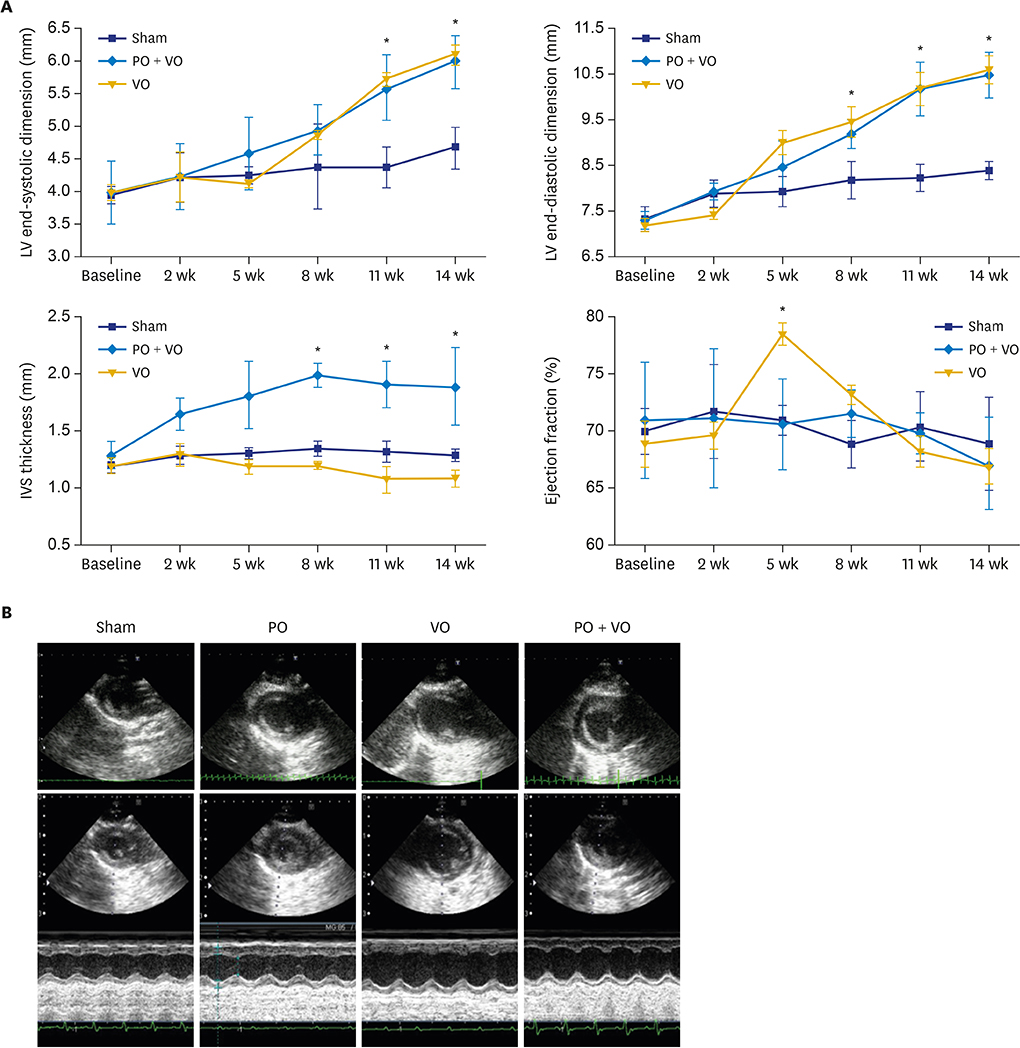
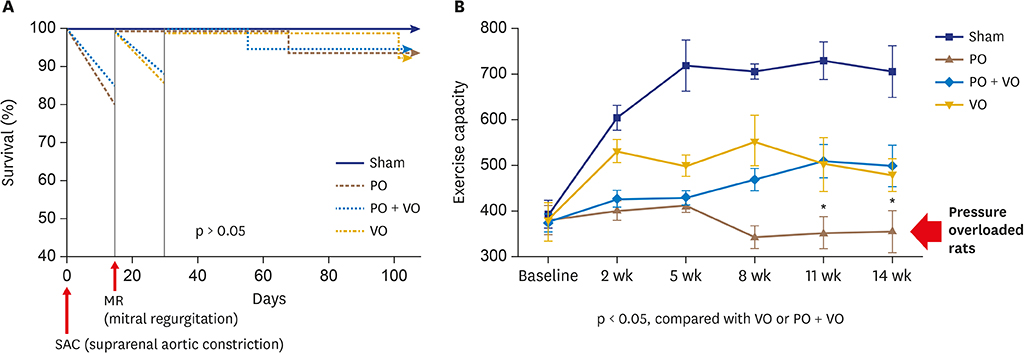
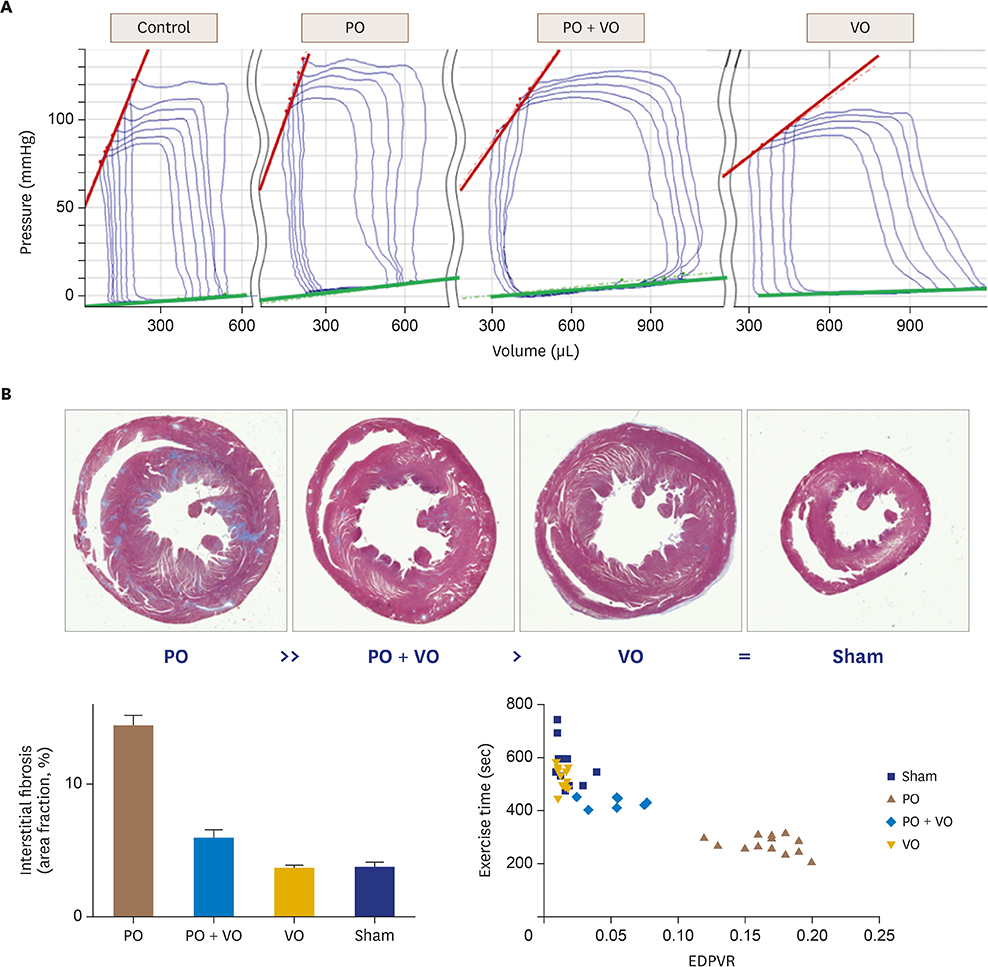
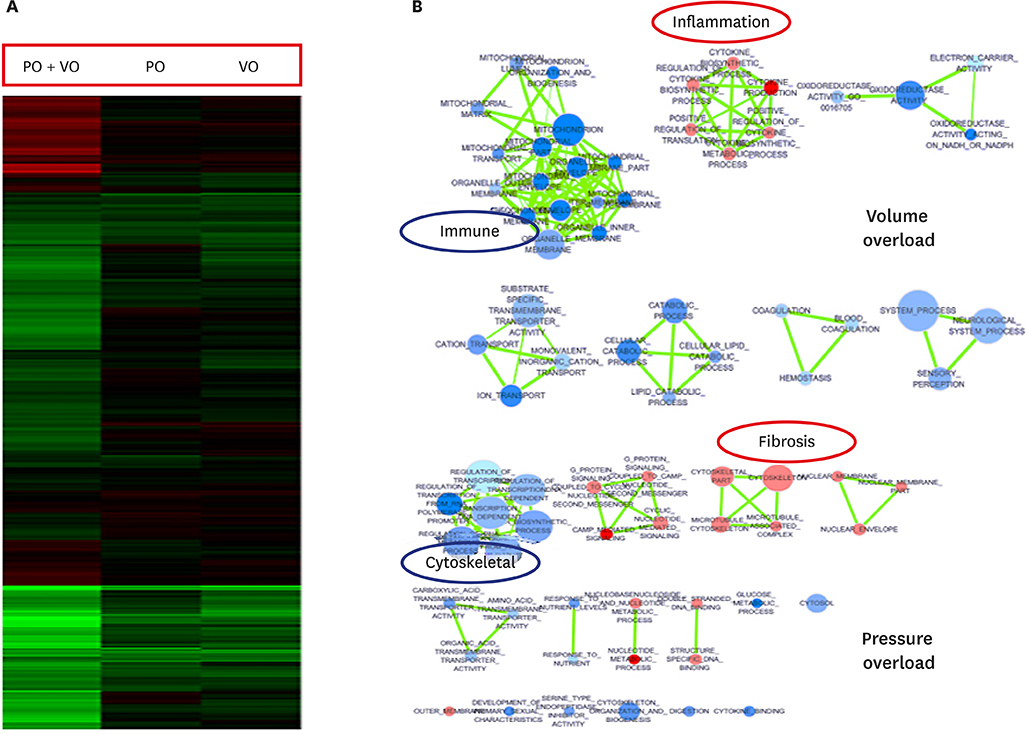
In hemodynamic analyses, the LV end-diastolic pressure and the end-diastolic pressure-volume relationship slope were greater in the PO group than in the VO group. When we compared LV remodeling and exercise capacity, cardiac fibrosis and exercise intolerance developed in the PO group but not in the VO group (exercise duration, 434.0 ± 80.3 vs. 497.8 ± 49.0 seconds, p < 0.05, respectively). Transcriptional profiling of cardiac apical tissues revealed that gene expression related to the inflammatory response and cellular signaling pathways were significantly enriched in the VO group, whereas cardiac fibrosis, cytoskeletal pathway and G-protein signaling genes were enriched in the PO group.
CONCLUSIONS
We found that many genes were regulated in PO, VO or both, and that there were different regulation patterns by cardiac remodeling. Cardiac fibrosis and cytoskeletal pathway were important pathways in the PO group and influenced exercise capacity. Cardiac fibrosis influences exercise capacity before LV function is reduced.
MeSH Terms
Figure
Cited by 1 articles
-
Unraveling the Mechanism of Cardiac Remodeling in Overloaded Heart: From Experiment to Theory
In-Jeong Cho
J Cardiovasc Imaging. 2019;27(1):64-65. doi: 10.4250/jcvi.2019.27.e10.
Reference
-
1. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011; 4:98–108.2. Yang DK, Choi BY, Lee YH, et al. Gene profiling during regression of pressure overload-induced cardiac hypertrophy. Physiol Genomics. 2007; 30:1–7.
Article3. Miyazaki H, Oka N, Koga A, Ohmura H, Ueda T, Imaizumi T. Comparison of gene expression profiling in pressure and volume overload-induced myocardial hypertrophies in rats. Hypertens Res. 2006; 29:1029–1045.
Article4. Phrommintikul A, Tran L, Kompa A, et al. Effects of a Rho kinase inhibitor on pressure overload induced cardiac hypertrophy and associated diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2008; 294:H1804–H1814.
Article5. Kim KH, Kim YJ, Lee SP, et al. Survival, exercise capacity, and left ventricular remodeling in a rat model of chronic mitral regurgitation: serial echocardiography and pressure-volume analysis. Korean Circ J. 2011; 41:603–611.
Article6. Kim KH, Kim YJ, Ohn JH, et al. Long-term effects of sildenafil in a rat model of chronic mitral regurgitation: benefits of ventricular remodeling and exercise capacity. Circulation. 2012; 125:1390–1401.7. Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008; 3:1422–1434.
Article8. Doi R, Masuyama T, Yamamoto K, et al. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J Hypertens. 2000; 18:111–120.9. Kai H, Kuwahara F, Tokuda K, Imaizumi T. Diastolic dysfunction in hypertensive hearts: roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res. 2005; 28:483–490.
Article10. Olson JJ, Costa SP, Young CE, Palac RT. Early mitral filling/diastolic mitral annular velocity ratio is not a reliable predictor of left ventricular filling pressure in the setting of severe mitral regurgitation. J Am Soc Echocardiogr. 2006; 19:83–87.
Article11. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63:e57–e185.12. Moore-Morris T, Guimarães-Camboa N, Banerjee I, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014; 124:2921–2934.
Article13. Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011; 89:265–272.
Article14. Wang BW, Wu GJ, Cheng WP, Shyu KG. MicroRNA-208a increases myocardial fibrosis via endoglin in volume overloading heart. PLoS One. 2014; 9:e84188.
Article15. Ulasova E, Gladden JD, Chen Y, et al. Loss of interstitial collagen causes structural and functional alterations of cardiomyocyte subsarcolemmal mitochondria in acute volume overload. J Mol Cell Cardiol. 2011; 50:147–156.
Article16. Ryan TD, Rothstein EC, Aban I, et al. Left ventricular eccentric remodeling and matrix loss are mediated by bradykinin and precede cardiomyocyte elongation in rats with volume overload. J Am Coll Cardiol. 2007; 49:811–821.
Article17. Chen YW, Pat B, Gladden JD, et al. Dynamic molecular and histopathological changes in the extracellular matrix and inflammation in the transition to heart failure in isolated volume overload. Am J Physiol Heart Circ Physiol. 2011; 300:H2251–H2260.
Article18. Stewart JA Jr, Wei CC, Brower GL, et al. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2003; 35:311–319.
Article19. Franz M, Berndt A, Altendorf-Hofmann A, et al. Serum levels of large tenascin-C variants, matrix metalloproteinase-9, and tissue inhibitors of matrix metalloproteinases in concentric versus eccentric left ventricular hypertrophy. Eur J Heart Fail. 2009; 11:1057–1062.
Article20. Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol. 2002; 39:1384–1391.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epigallocatechin-3 gallate prevents cardiac hypertrophy induced by pressure overload in rats
- Comparing the Effects of Carvedilol Enantiomers on Regression of Established Cardiac Hypertrophy Induced by Pressure Overload
- The Relationship of Pulmonary Regurgitation and Exercise Performance after Repair of Tetralogy of Fallot
- Intensive management of acute right heart failure
- Survival, Exercise Capacity, and Left Ventricular Remodeling in a Rat Model of Chronic Mitral Regurgitation: Serial Echocardiography and Pressure-Volume Analysis