Chonnam Med J.
2019 Jan;55(1):20-24. 10.4068/cmj.2019.55.1.20.
Comparison of I-131 Biokinetics after Recombinant Human TSH Stimulation and Thyroid Hormone Withdrawal Measured by External Detector in Patients with Differentiated Thyroid Cancer
- Affiliations
-
- 1Molecular Radiotherapy & Nuclear Medicine, Docrates Cancer Center, Helsinki, Finland. kalevi.kairemo@docrates.com
- 2Finland Radiation Physics, Docrates Cancer Center, Helsinki, Finland.
- 3Department of Nuclear Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
- 4Department of Nuclear Medicine, Chonnam National University Medical School, Gwangju, Korea. kalevi. henryhsbom@gmail.com
- KMID: 2432251
- DOI: http://doi.org/10.4068/cmj.2019.55.1.20
Abstract
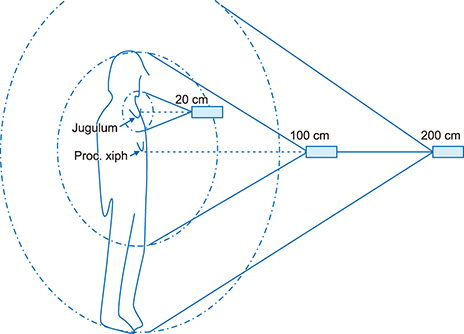
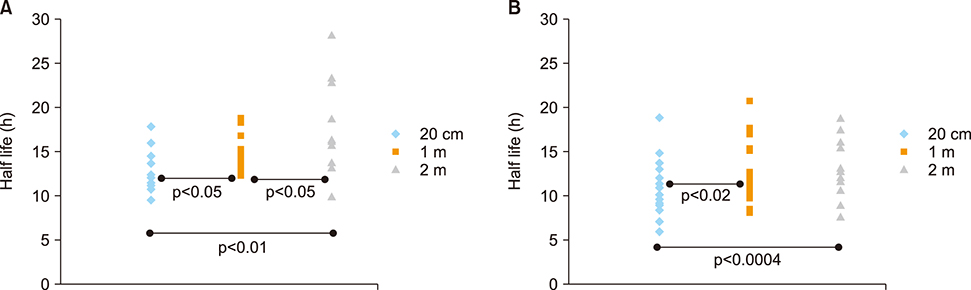
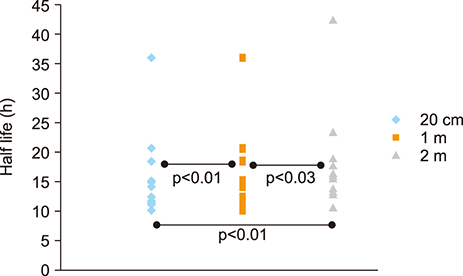
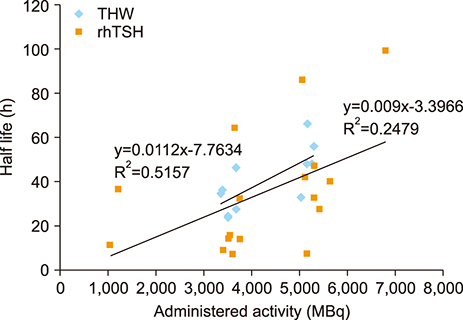
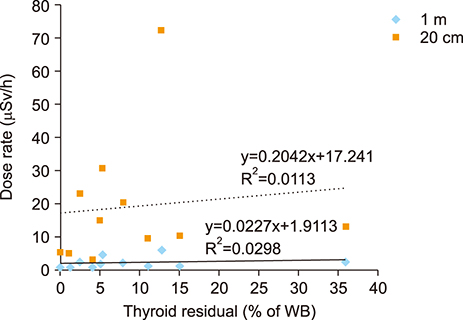
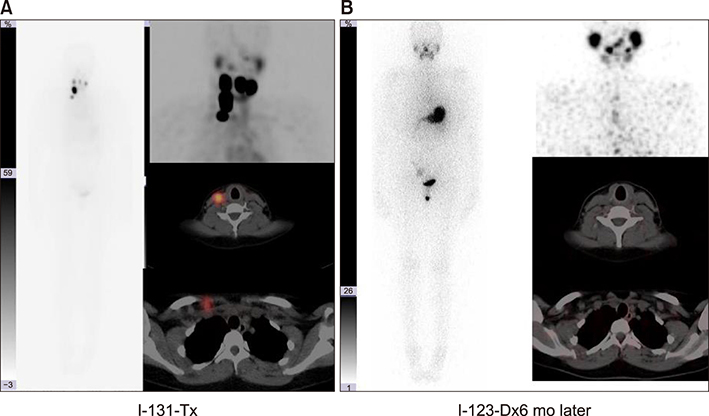
- The aim of this study was to compare radioactive iodine (I-131) biokinetics after recombinant human TSH stimulation (rhTSH) and thyroid hormone withdrawal (THW) in patients with differentiated thyroid cancer (DTC). External effective dose rates were measured using external detectors and imaged quantitatively at the time of discharge from the isolation wards. We retrospectively analyzed 32 patients who had been diagnosed with DTC, papillary or follicular, and underwent remnant ablation after either rhTSH stimulation (n=22) or THW (n=10). The uptake of I-131 by remnant thyroid tissue was measured from 20.0 cm, 100.0 cm and 200.0 cm distances using a handheld external detector. The remnant thyroid tissue measured by the whole body images two to five days from administration was 10.7+26.0% (range 0.5 to 60.0%). The values measured at 20 cm were best correlated to the thyroid residual uptake measured by SPECT/CT. The half-lives of I-131washout (T1/2) in rhTSH group measured by external detector were shorter than those of THW group. T1/2 becomes longer when it was measured over longer distances. They were 10.9, 12.3 and 13.1 hours at distances of 20, 100, and 200 cm in rhTSH group, respectively. The TWH group showed 12.8, 14.9 and 17.7 hours, respectively. We conclude that I-131 biokinetics can be measured by external detector after high dose I-131 therapy for DTC. It showed that washout of I-131 was faster after rhTSH stimulation than THW, and slower in patients with distant metastasis than those without metastasis.
Keyword
MeSH Terms
Figure
Reference
-
1. Beierwaltes WH, Rabbani R, Dmuchowski C, Lloyd RV, Eyre P, Mallette S. An analysis of “ablation of thyroid remnants” with I-131 in 511 patients from 1947-1984: experience at University of Michigan. J Nucl Med. 1984; 25:1287–1293.2. McCowen KD, Adler RA, Ghaed N, Verdon T, Hofeldt FD. Low dose radioiodide thyroid ablation in postsurgical patients with thyroid cancer. Am J Med. 1976; 61:52–58.
Article3. Kayano D, Taki J, Inaki A, Wakabayashi H, Nakamura A, Fukuoka M, et al. Thyroid hormone replacement one day before (131)I therapy in patients with well-differentiated thyroid cancer. Asia Ocean J Nucl Med Biol. 2013; 1:20–26.4. Bal CS, Padhy AK. Radioiodine remnant ablation: a critical review. World J Nucl Med. 2015; 14:144–155.
Article5. Jabin Z, Kwon SY, Bom HS, Lin Y, Yang K, Inaki A, et al. Clinico-social factors to choose radioactive iodine dose in differentiated thyroid cancer patients: an Asian survey. Nucl Med Commun. 2018; 39:283–289.
Article6. Wakabayashi H, Taki J, Inaki A, Toratani A, Kayano D, Kinuya S. Extremity radioactive iodine uptake on post-therapeutic whole body scan in patients with differentiated thyroid cancer. Asia Ocean J Nucl Med Biol. 2015; 3:26–34.7. Duntas LH, Cooper DS. Review on the occasion of a decade of recombinant human TSH: prospects and novel uses. Thyroid. 2008; 18:509–516.
Article8. Robbins RJ, Tuttle RM, Sonenberg M, Shaha A, Sharaf R, Robbins H, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin. Thyroid. 2001; 11:865–869.
Article9. Berg G, Lindstedt G, Suurküla M, Jansson S. Radioiodine ablation and therapy in differentiated thyroid cancer under stimulation with recombinant human thyroid-stimulating hormone. J Endocrinol Invest. 2002; 25:44–52.
Article10. Hänscheid H, Lassmann M, Luster M, Thomas SR, Pacini F, Ceccarelli C, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. 2006; 47:648–654.11. Hong CM, Kim CY, Son SH, Jung JH, Lee CH, Jeong JH, et al. I-131 biokinetics of remnant normal thyroid tissue and residual thyroid cancer in patients with differentiated thyroid cancer: comparison between recombinant human TSH administration and thyroid hormone withdrawal. Ann Nucl Med. 2017; 31:582–589.
Article12. Schlumberger M, Lacroix L, Russo D, Filetti S, Bidart JM. Defects in iodide metabolism in thyroid cancer and implications for the follow-up and treatment of patients. Nat Clin Pract Endocrinol Metab. 2007; 3:260–269.
Article13. Taïeb D, Sebag F, Farman-Ara B, Portal T, Baumstarck-Barrau K, Fortanier C, et al. Iodine biokinetics and radioiodine exposure after recombinant human thyrotropin-assisted remnant ablation in comparison with thyroid hormone withdrawal. J Clin Endocrinol Metab. 2010; 95:3283–3290.
Article14. Hung BT, Huang SH, Huang YE, Wang PW. Appropriate time for post-therapeutic I-131 whole body scan. Clin Nucl Med. 2009; 34:339–342.
Article15. Kairemo K. Thyroid doses and skin contaminations of radioiodine. J Nucl Med Radiat Ther. 2015; 6:244.
Article16. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:418–428.
Article17. Samaan NA, Maheshwari YK, Nader S, Hill CS Jr, Schultz PN, Haynie TP, et al. Impact of therapy for differentiated carcinoma of the thyroid: an analysis of 706 cases. J Clin Endocrinol Metab. 1983; 56:1131–1138.
Article18. Simpson WJ, Panzarella T, Carruthers JS, Gospodarowicz MK, Sutcliffe SB. Papillary and follicular thyroid cancer: impact of treatment in 1578 patients. Int J Radiat Oncol Biol Phys. 1988; 14:1063–1075.
Article19. Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC. Clinical review 170: a systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004; 89:3668–3676.
Article20. Byun BH, Kwon SY, Chong A, Kim J, Yoo SW, Min JJ, et al. Both F-18 FDG-avidity and malignant shape of cervical lymph nodes on PET/CT after total thyroidectomy predict resistance to high-dose I-131 therapy in patients with papillary thyroid cancer. Asia Ocean J Nucl Med Biol. 2013; 1:6–13.21. Chereau N, Trésallet C, Noullet S, Godiris-Petit G, Tissier F, Leenhardt L, et al. Prognosis of papillary thyroid carcinoma in elderly patients after thyroid resection: a retrospective cohort analysis. Medicine (Baltimore). 2016; 95:e5450.22. Molenaar RJ, Sidana S, Radivoyevitch T, Advani AS, Gerds AT, Carraway HE, et al. Risk of hematologic malignancies after radioiodine treatment of well-differentiated thyroid cancer. J Clin Oncol. 2018; 36:1831–1839.
Article23. Tulchinsky M, Baum RP, Bennet KG, Freeman LM, Jong I, Kairemo K, et al. Well-founded recommendations for radioactive iodine treatment of differentiated thyroid cancer require balanced study of benefits and harms. J Clin Oncol. 2018; 36:1887–1888.
Article24. Tulchinsky M, Binse I, Campennì A, Dizdarevic S, Giovanella L, Jong I, et al. Radioactive iodine therapy for differentiated thyroid cancer: lessons from confronting controversial literature on risks for secondary malignancy. J Nucl Med. 2018; 59:723–725.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Retrospective Review of the Effectiveness of Recombinant Human TSH-Aided Radioiodine Treatment of Differentiated Thyroid Carcinoma
- Comparison of I-131 Scintigraphy , T1-201 Scintigraphy , and Serum Thyroglobulin in the Postoperative Follow-Up of Differentiated Thyroid Cancer
- Clinical Significance of Diffuse Intrathoracic Uptake on Post-Therapy I-131 Scans in Thyroid Cancer Patients
- TSH Suppression after Differentiated Thyroid Cancer Surgery and Osteoporosis
- Clinical Factors Associated with Quality of Life in Patients with Thyroid Cancer