Pediatr Gastroenterol Hepatol Nutr.
2019 Jan;22(1):63-71. 10.5223/pghn.2019.22.1.63.
Dosage-Related Prebiotic Effects of Inulin in Formula-Fed Infants
- Affiliations
-
- 1Department of Child Health, Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia. hoswari@gmail.com
- 2Research Task Force, Indonesian Pediatric Society (IDAI), Jakarta, Indonesia.
- 3Department of Pediatrics, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia.
- 4Danone Nutricia Early Life Nutrition R&D, Jakarta, Indonesia.
- 5Nutricia Research, Danone Nutricia Early Life Nutrition, Singapore, Singapore.
- KMID: 2430917
- DOI: http://doi.org/10.5223/pghn.2019.22.1.63
Abstract
- PURPOSE
The aim of this study was to identify the minimally meaningful dosage of inulin leading to a prebiotic effect in Indonesian infants.
METHODS
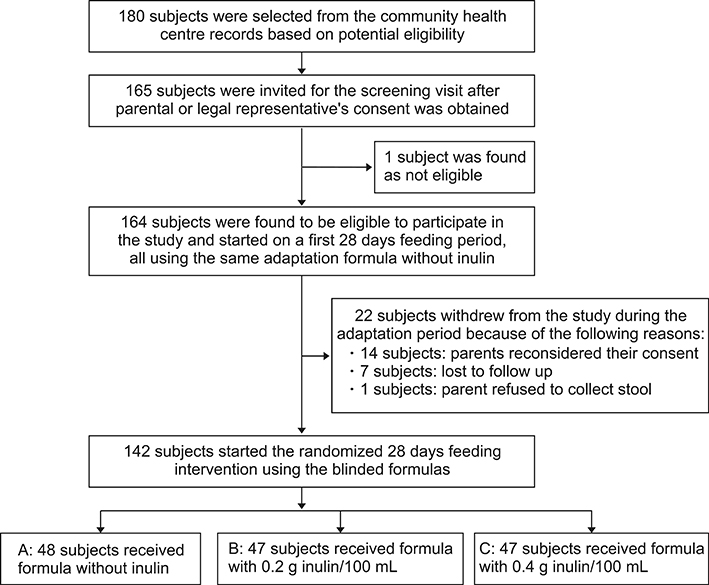
In a randomized controlled double-blinded, parallel, 3-arm intervention study, 164 healthy formula-fed infants aged 3 to 5 months first obtained formula-A (without inulin) during a 4-week adaptation period. Subsequently, 142 subjects were subjected to a 4-week feeding period by administering either formula-A (no inulin), formula-B (0.2 g/100 mL inulin) or formula-C (0.4 g/100 mL inulin). The primary outcome parameter was %-bifidobacteria in faecal samples determined using quantitative polymerase chain reaction analyses. Secondary outcome parameters were faecal %-lactobacilli, pH and stool frequency, and consistency. Growth and tolerance/adverse effects were recorded as safety parameters.
RESULTS
Typical %-bifidobacteria and %-lactobacilli at the end of the adaptation period in the study population were 14% and 2%, respectively. For faecal pH, significant differences between formula groups A vs. C and A vs. B were found at the end of the intervention period. Testing for differences in faecal %-bifidobacteria and %-lactobacilli between groups was hampered by non-normal data set distributions; no statistically significant differences were obtained. Comparisons within groups revealed that only in formula group C, all the three relevant parameters exhibited a significant effect with an increase in faecal %-bifidobacteria and %-lactobacilli and a decrease in pH.
CONCLUSION
A consistent prebiotic effect along with a decrease in pH and increase in %-bifidobacteria and %-lactobacilli was found only in the group administered 0.4 g inulin/100 mL.
MeSH Terms
Figure
Reference
-
1. Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010; 104:Suppl 2. S1–S63.
Article2. Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2011; 52:238–250.
Article3. Moro G, Minoli I, Mosca M, Fanaro S, Jelinek J, Stahl B, et al. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J Pediatr Gastroenterol Nutr. 2002; 34:291–295.
Article4. Paineau D, Respondek F, Menet V, Sauvage R, Bornet F, Wagner A. Effects of short-chain fructooligosaccharides on faecal bifidobacteria and specific immune response in formula-fed term infants: a randomized, double-blind, placebo-controlled trial. J Nutr Sci Vitaminol (Tokyo). 2014; 60:167–175.
Article5. Veereman-Wauters G, Staelens S, Van de Broek H, Plaskie K, Wesling F, Roger LC, et al. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J Pediatr Gastroenterol Nutr. 2011; 52:763–771.
Article6. Fanaro S, Marten B, Bagna R, Vigi V, Fabris C, Peña-Quintana L, et al. Galacto-oligosaccharides are bifidogenic and safe at weaning: a double-blind randomized multicenter study. J Pediatr Gastroenterol Nutr. 2009; 48:82–88.
Article7. Scalabrin DM, Mitmesser SH, Welling GW, Harris CL, Marunycz JD, Walker DC, et al. New prebiotic blend of polydextrose and galacto-oligosaccharides has a bifidogenic effect in young infants. J Pediatr Gastroenterol Nutr. 2012; 54:343–352.
Article8. Firmansyah A, Chongviriyaphan N, Dillon DH, Khan NC, Morita T, Tontisirin K, et al. Fructans in the first 1000 days of life and beyond, and for pregnancy. Asia Pac J Clin Nutr. 2016; 25:652–675.9. K W Yap W, Mohamed S, Husni Jamal M, Diederick M, Manap YA. Changes in infants faecal characteristics and microbiota by inulin supplementation. J Clin Biochem Nutr. 2008; 43:159–166.
Article10. Kim SH, Lee DH, Meyer D. Supplementation of baby formula with native inulin has a prebiotic effect in formula-fed babies. Asia Pac J Clin Nutr. 2007; 16:172–177.11. Lugonja NM, Martinov OB, Rasovic MR, Spasic SD, Gojgic GDj, Vrvic MM. A comparative investigation of an in vitro and clinical test of the bifidogenic effect of an infant formula. J Clin Biochem Nutr. 2010; 47:208–216.
Article12. Yap KW, Mohamed S, Yazid AM, Maznah I, Meyer DM. Dose-response effects of inulin on the faecal short-chain fatty acids content and mineral absorption of formulafed infants. Nutr Food Sci. 2005; 35:208–219.
Article13. Loo JV. Inulin-type fructans as prebiotics. In : Gibson GR, Rastall RA, editors. Prebiotics: development and application. Chichester: John Wiley & Sons;2006. p. 57–100.14. van de Wiele T, Boon N, Possemiers S, Jacobs H, Verstraete W. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J Appl Microbiol. 2007; 102:452–460.
Article15. Bonnema AL, Kolberg LW, Thomas W, Slavin JL. Gastrointestinal tolerance of chicory inulin products. J Am Diet Assoc. 2010; 110:865–868.
Article16. Oswari H, Prayitno L, Dwipoerwantoro PG, Firmansyah A, Makrides M, Lawley B, et al. Comparison of stool microbiota compositions, stool alpha1-antitrypsin and calprotectin concentrations, and diarrhoeal morbidity of Indonesian infants fed breast milk or probiotic/prebiotic-supplemented formula. J Paediatr Child Health. 2013; 49:1032–1039.
Article17. Yap GC, Chee KK, Hong PY, Lay C, Satria CD, Sumadiono , et al. Evaluation of stool microbiota signatures in two cohorts of Asian (Singapore and Indonesia) newborns at risk of atopy. BMC Microbiol. 2011; 11:193.
Article18. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997; 32:920–924.
Article19. Wang Y, Chen H. Use of percentiles and Z-scores in anthropometry. In : Preedy V, editor. Handbook of anthropometry : physical measures of human form in health and disease. New York: Springer;2012. p. 29–48.20. Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000; 30:61–67.
Article21. Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K, et al. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr. 2005; 40:36–42.
Article22. Scholtens PA, Alles MS, Bindels JG, van der Linde EG, Tolboom JJ, Knol J. Bifidogenic effects of solid weaning foods with added prebiotic oligosaccharides: a randomised controlled clinical trial. J Pediatr Gastroenterol Nutr. 2006; 42:553–559.
Article23. Haarman M, Knol J. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. 2005; 71:2318–2324.
Article24. Cuello-Garcia CA, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Morgano GP, Zhang Y, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): prebiotics. World Allergy Organ J. 2016; 9:10.
Article25. Boehm G, Jelinek J, Knol J, M'Rabet L, Stahl B, Vos P, et al. Prebiotics and immune responses. J Pediatr Gastroenterol Nutr. 2004; 39:Suppl 3. S772–S773.
Article26. Bakker-Zierikzee AM, Alles MS, Knol J, Kok FJ, Tolboom JJ, Bindels JG. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr. 2005; 94:783–790.
Article27. Euler AR, Mitchell DK, Kline R, Pickering LK. Prebiotic effect of fructo-oligosaccharide supplemented term infant formula at two concentrations compared with unsupplemented formula and human milk. J Pediatr Gastroenterol Nutr. 2005; 40:157–164.
Article28. Nakamura N, Gaskins HR, Collier CT, Nava GM, Rai D, Petschow B, et al. Molecular ecological analysis of fecal bacterial populations from term infants fed formula supplemented with selected blends of prebiotics. Appl Environ Microbiol. 2009; 75:1121–1128.
Article29. Kementerian Kesehatan Republik Indonesia. Data dan informasi: profil kesehatan Indonesia 2017 [Internet]. Jakarta: Kementerian Kesehatan Republik Indonesia;2018. cited 2018 Aug 16. Available from: http://www.depkes.go.id/resources/download/pusdatin/profil-kesehatan-indonesia/Data-dan-Informasi_Profil-Kesehatan-Indonesia-2017.pdf.30. Tuohy KM, Finlay RK, Wynne AG, Gibson GR. A human volunteer study on the prebiotic effects of HP-inulin—faecal bacteria enumerated using fluorescent in situ hybridisation (FISH). Anaerobe. 2001; 7:113–118.
Article31. Harmsen HJM, Raangs GC, Franks AH, Wildeboer-Veloo ACM, Welling GW. The effect of the prebiotic inulin and the probiotic bifidobacterium longum on the fecal microflora of healthy volunteers measured by FISH and DGGE. Microb Ecol Health Dis. 2002; 14:211–220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Hematologic and Serum Biochemistric Values at 1 Month of Age between Breast-fed and Formula-fed Infants
- A Longitudinal Study of Calcium and Phosphorus Intakes of Korean Infants from 1 to 3 Months in Breast-Fed vs Formula-Fed Infants
- Growth Patterns of Breast Fed and Formula Fed Infants
- Acid Steatocrit in Korean Infants
- Comparison of the Effectiveness of Phototherapy for Nonhemolytic Hyperbilirubinemia in Breast-fed and Formula-fed Infants