J Gastric Cancer.
2018 Dec;18(4):368-378. 10.5230/jgc.2018.18.e36.
The Fibrinogen to Mean Platelet Volume Ratio Can Predict Overall Survival of Patients with Non-Metastatic Gastric Cancer
- Affiliations
-
- 1Department of Gastrointestinal Surgery, Harbin Medical University Cancer Hospital, Harbin, China. xyw801@163.com
- KMID: 2429883
- DOI: http://doi.org/10.5230/jgc.2018.18.e36
Abstract
- PURPOSE
Fibrinogen and platelets have been reported to play important roles in tumorigenesis and cancer progression. The aim of this research was to investigate the combination of functions of fibrinogen, platelets, and mean platelet volume (MPV) in predicting the survival of patients with gastric cancer (GC).
MATERIALS AND METHODS
A retrospective study was conducted with 1,946 patients with GC and 299 patients with benign gastric tumor to analyze their fibrinogen, platelet, and MPV levels, and other clinicopathological characteristics along with their prognoses. Several indicators were evaluated along with fibrinogen, platelets, and MPV and their prognostic abilities were assessed. Univariate and multivariate survival analyses were conducted to determine the independent risk factors for overall survival.
RESULTS
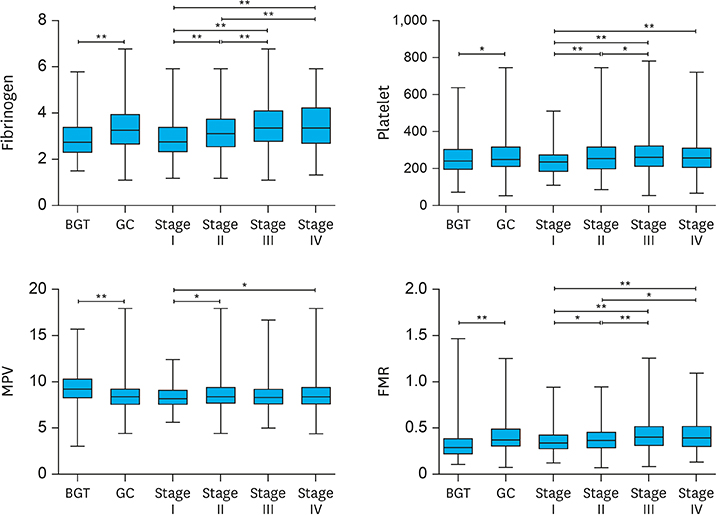
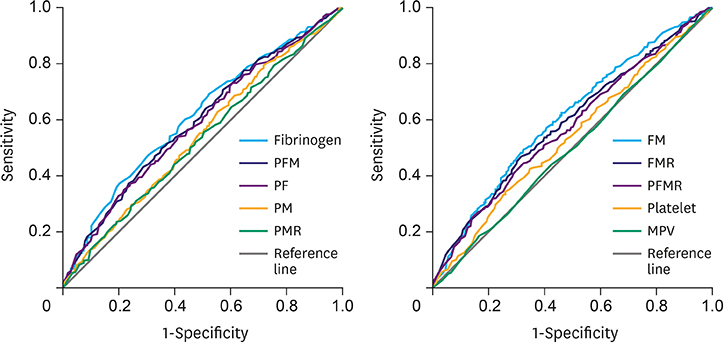
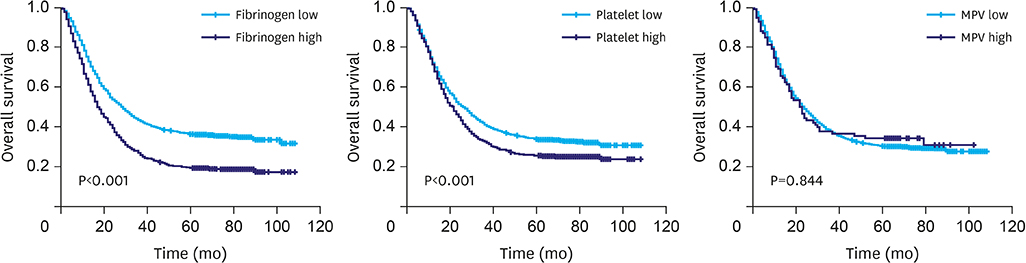
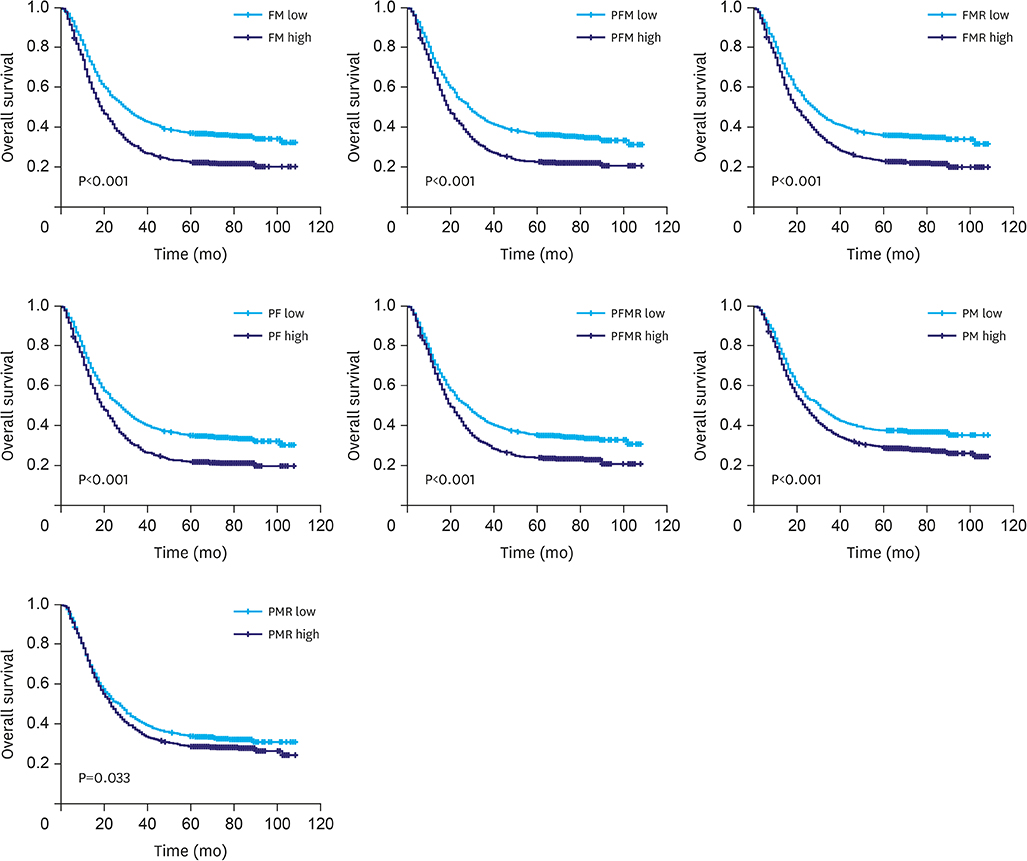
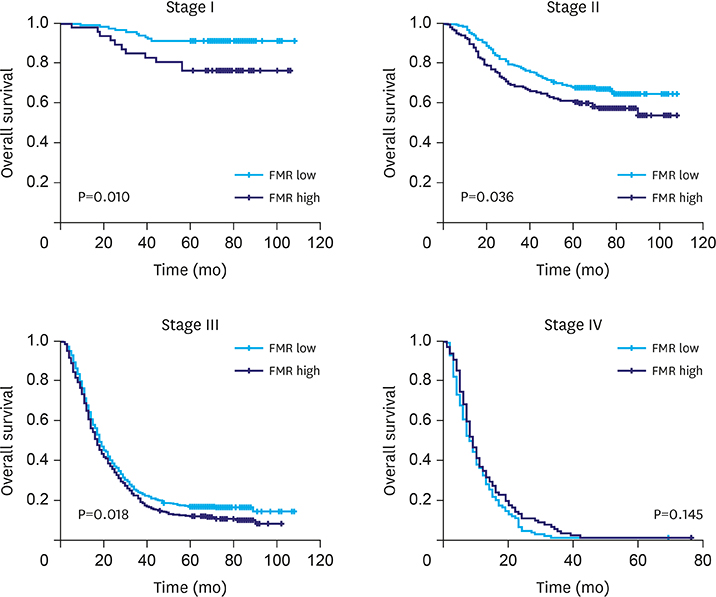
Increased levels of fibrinogen, platelets, and MPV were observed with the progress of the GC stages. Elevated fibrinogen, platelets, and the combined indicators, including fibrinogen*MPV (FM), platelet*fibrinogen*MPV (PFM), fibrinogen/MPV (FMR), platelet*fibrinogen (PF), platelet*fibrinogen/MPV (PFMR), platelet*MPV (PM), and platelet/MPV (PMR), foreboded poor prognosis. Meanwhile fibrinogen and FMR can be considered as independent risk factors for overall survival in patients with non-metastatic GC. But these indicators can hardly predict survival of patients in stage IV.
CONCLUSIONS
Elevated fibrinogen, platelets, and MPV levels were in accordance with advanced stages, and fibrinogen, platelet, and MPV, in combination, can be used to predict survival of patients with non-metastatic GC. FMR was an independent prognostic factor for overall survival of patients with GC.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–132.
Article3. Wang WH, Huang JQ, Zheng GF, Lam SK, Karlberg J, Wong BC. Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2003; 95:1784–1791.
Article4. Mroczko B, Groblewska M, Łukaszewicz-Zajac M, Bandurski R, Kedra B, Szmitkowski M. Pre-treatment serum and plasma levels of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) in gastric cancer patients. Clin Chem Lab Med. 2009; 47:1133–1139.
Article5. Zheng S, Shen J, Jiao Y, Liu Y, Zhang C, Wei M, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009; 100:859–865.
Article6. Takagi S, Sato S, Oh-hara T, Takami M, Koike S, Mishima Y, et al. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS One. 2013; 8:e73609.
Article7. Yu X, Hu F, Yao Q, Li C, Zhang H, Xue Y. Serum fibrinogen levels are positively correlated with advanced tumor stage and poor survival in patients with gastric cancer undergoing gastrectomy: a large cohort retrospective study. BMC Cancer. 2016; 16:480.
Article8. Lian L, Xia YY, Zhou C, Shen XM, Li XL, Han SG, et al. Mean platelet volume predicts chemotherapy response and prognosis in patients with unresectable gastric cancer. Oncol Lett. 2015; 10:3419–3424.
Article9. Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000; 45:23–41.
Article10. Suzuki T, Shimada H, Nanami T, Oshima Y, Yajima S, Ito M, et al. Hyperfibrinogenemia is associated with inflammatory mediators and poor prognosis in patients with gastric cancer. Surg Today. 2016; 46:1394–1401.
Article11. Song S, Li C, Li S, Gao H, Lan X, Xue Y. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther. 2017; 10:3145–3154.
Article12. Inagaki N, Kibata K, Tamaki T, Shimizu T, Nomura S. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer. 2014; 83:97–101.
Article13. Kurt M, Onal IK, Sayilir AY, Beyazit Y, Oztas E, Kekilli M, et al. The role of mean platelet volume in the diagnosis of hepatocellular carcinoma in patients with chronic liver disease. Hepatogastroenterology. 2012; 59:1580–1582.14. Zhu LR, Li J, Chen P, Jiang Q, Tang XP. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and prognosis for small cell lung cancer patients. Clin Transl Oncol. 2016; 18:178–188.
Article15. Sheng L, Luo M, Sun X, Lin N, Mao W, Su D. Serum fibrinogen is an independent prognostic factor in operable nonsmall cell lung cancer. Int J Cancer. 2013; 133:2720–2725.
Article16. Matsuda S, Takeuchi H, Kawakubo H, Fukuda K, Nakamura R, Takahashi T, et al. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: comparison with the Glasgow prognostic score. Ann Surg Oncol. 2015; 22:302–310.
Article17. Mei Y, Zhao S, Lu X, Liu H, Li X, Ma R. Clinical and prognostic significance of preoperative plasma fibrinogen levels in patients with operable breast cancer. PLoS One. 2016; 11:e0146233.
Article18. Yamaguchi T, Yamamoto Y, Yokota S, Nakagawa M, Ito M, Ogura T. Involvement of interleukin-6 in the elevation of plasma fibrinogen levels in lung cancer patients. Jpn J Clin Oncol. 1998; 28:740–744.19. Mikami J, Kurokawa Y, Takahashi T, Miyazaki Y, Yamasaki M, Miyata H, et al. Antitumor effect of antiplatelet agents in gastric cancer cells: an in vivo and in vitro study. Gastric Cancer. 2016; 19:817–826.
Article20. Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res. 2003; 9:383–390.21. Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005; 105:178–185.
Article22. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011; 11:123–134.
Article23. Yu LX, Yan L, Yang W, Wu FQ, Ling Y, Chen SZ, et al. Platelets promote tumour metastasis via interaction between TLR4 and tumour cell-released high-mobility group box1 protein. Nat Commun. 2014; 5:5256.
Article24. Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007; 357:2482–2494.
Article25. Oge T, Yalcin OT, Ozalp SS, Isikci T. Platelet volume as a parameter for platelet activation in patients with endometrial cancer. J Obstet Gynaecol. 2013; 33:301–304.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Metastatic Gastric Cancer
- High Preoperative Fibrinogen and Systemic Inflammation Response Index (F-SIRI) Predict Unfavorable Survival of Resectable Gastric Cancer Patients
- Systemic Inflammatory Biomarkers, Especially Fibrinogen to Albumin Ratio, Predict Prognosis in Patients with Pancreatic Cancer
- Comparison of Nodal Staging, of UICC TNM and Japanese Classification, and Prognostic Nodal Grouping of UICC N3M0 in Advanced Gastric Cancer
- Role of Metastasectomy on Overall Survival of Patients with Metastatic Gastric Cancer