J Periodontal Implant Sci.
2018 Dec;48(6):373-382. 10.5051/jpis.2018.48.6.373.
Effects of various prophylactic procedures on titanium surfaces and biofilm formation
- Affiliations
-
- 1Research Institute on Terrestrial Ecosystems, National Research Council, Naples, Italy. anna.disalle@cnr.it
- 2Department of Neurosciences, Reproductive and Odontostomatological Sciences, University of Naples Federico II, Naples, Italy.
- 3I. M. Sechenov First Moscow State Medical University, Institute of Dentistry, Moscow, Russia.
- KMID: 2429865
- DOI: http://doi.org/10.5051/jpis.2018.48.6.373
Abstract
- PURPOSE
The aim of this study was to evaluate the effects of various prophylactic treatments of titanium implants on bacterial biofilm formation, correlating surface modifications with the biofilms produced by Pseudomonas aeruginosa PAO1, Staphylococcus aureus, and bacteria isolated from saliva.
METHODS
Pure titanium disks were treated with various prophylactic procedures, and atomic force microscopy (AFM) was used to determine the degree to which surface roughness was modified. To evaluate antibiofilm activity, we used P. aeruginosa PAO1, S. aureus, and saliva-isolated Streptococcus spp., Bacteroides fragilis, and Staphylococcus epidermidis.
RESULTS
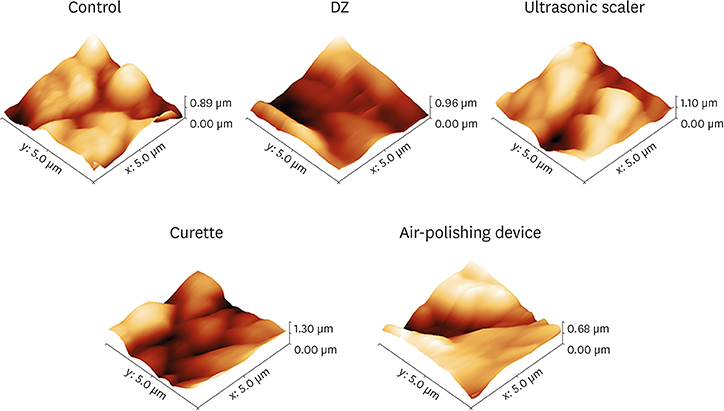
AFM showed that the surface roughness increased after using the air-polishing device and ultrasonic scaler, while a significant reduction was observed after using a curette or polishing with Detartrine ZTM (DZ) abrasive paste. In addition, we only observed a significant (P < 0.01) reduction in biofilm formation on the DZ-treated implant surfaces.
CONCLUSION
In this study, both AFM and antibiofilm analyses indicated that using DZ abrasive paste could be considered as the prophylactic procedure of choice for managing peri-implant lesions and for therapy-resistant cases of periodontitis.
MeSH Terms
Figure
Reference
-
1. Salvi GE, Cosgarea R, Sculean A. Prevalence and mechanisms of peri-implant diseases. J Dent Res. 2017; 96:31–37.
Article2. Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015; 42:Suppl 16. S158–S171.
Article3. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008; 35:Suppl. 286–291.
Article4. Albrektsson T, Buser D, Chen ST, Cochran D, DeBruyn H, Jemt T, et al. Statements from the Estepona consensus meeting on peri-implantitis, February 2–4, 2012. Clin Implant Dent Relat Res. 2012; 14:781–782.
Article5. Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013; 69:137–143.
Article6. Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007; 13:Suppl 4. 3–10.
Article7. Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers. 2017; 3:17030.
Article8. Chenicheri S, R U, Ramachandran R, Thomas V, Wood A. Insight into oral biofilm: primary, secondary and residual caries and phyto-challenged solutions. Open Dent J. 2017; 11:312–333.
Article9. Jepsen S, Berglundh T, Genco R, Aass AM, Demirel K, Derks J, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015; 42:Suppl 16. S152–S157.
Article10. Salvi GE, Ramseier CA. Efficacy of patient-administered mechanical and/or chemical plaque control protocols in the management of peri-implant mucositis. A systematic review. J Clin Periodontol. 2015; 42:Suppl 16. S187–S201.
Article11. Larsen T, Fiehn NE. Dental biofilm infections - an update. APMIS. 2017; 125:376–384.
Article12. Lin NJ. Biofilm over teeth and restorations: what do we need to know? Dent Mater. 2017; 33:667–680.
Article13. Subramani K, Jung RE, Molenberg A, Hammerle CH. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants. 2009; 24:616–626.14. Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010; 89:657–665.
Article15. Song F, Koo H, Ren D. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res. 2015; 94:1027–1034.
Article16. Suárez-López Del Amo F, Yu SH, Wang HL. Non-surgical therapy for peri-implant diseases: a systematic review. J Oral Maxillofac Res. 2016; 7:e13.
Article17. Ferraris S, Spriano S. Antibacterial titanium surfaces for medical implants. Mater Sci Eng C. 2016; 61:965–978.
Article18. Kreisler M, Kohnen W, Christoffers AB, Götz H, Jansen B, Duschner H, et al. In vitro evaluation of the biocompatibility of contaminated implant surfaces treated with an Er: YAG laser and an air powder system. Clin Oral Implants Res. 2005; 16:36–43.
Article19. Schwarz F, Ferrari D, Popovski K, Hartig B, Becker J. Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J Biomed Mater Res B Appl Biomater. 2009; 88:83–91.
Article20. Sahm N, Becker J, Santel T, Schwarz F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. J Clin Periodontol. 2011; 38:872–878.
Article21. Albertini M, López-Cerero L, O'Sullivan MG, Chereguini CF, Ballesta S, Ríos V, et al. Assessment of periodontal and opportunistic flora in patients with peri-implantitis. Clin Oral Implants Res. 2015; 26:937–941.
Article22. Canullo L, Rossetti PH, Penarrocha D. Identification of Enterococcus faecalis and Pseudomonas aeruginosa on and in implants in individuals with peri-implant disease: a cross-sectional study. Int J Oral Maxillofac Implants. 2015; 30:583–587.
Article23. Harris LG, Mead L, Müller-Oberländer E, Richards RG. Bacteria and cell cytocompatibility studies on coated medical grade titanium surfaces. J Biomed Mater Res A. 2006; 78:50–58.
Article24. Renvert S, Lindahl C, Renvert H, Persson GR. Clinical and microbiological analysis of subjects treated with Brånemark or AstraTech implants: a 7-year follow-up study. Clin Oral Implants Res. 2008; 19:342–347.
Article25. Mehl C, Kern M, Zimmermann A, Harder S, Huth S, Selhuber-Unkel C. Impact of cleaning procedures on adhesion of living cells to three abutment materials. Int J Oral Maxillofac Implants. 2017; 32:976–984.
Article26. Cafiero C, Aglietta M, Iorio-Siciliano V, Salvi GE, Blasi A, Matarasso S. Implant surface roughness alterations induced by different prophylactic procedures: an in vitro study. Clin Oral Implants Res. 2017; 28:e16–20.27. Chen CJ, Ding SJ, Chen CC. Effects of surface conditions of titanium dental implants on bacterial adhesion. Photomed Laser Surg. 2016; 34:379–388.
Article28. Ametrano G, D’Antò V, Di Caprio MP, Simeone M, Rengo S, Spagnuolo G. Effects of sodium hypochlorite and ethylenediaminetetraacetic acid on rotary nickel-titanium instruments evaluated using atomic force microscopy. Int Endod J. 2011; 44:203–209.
Article29. Spagnuolo G, Ametrano G, D’Antò V, Rengo C, Simeone M, Riccitiello F, et al. Effect of autoclaving on the surfaces of TiN-coated and conventional nickel-titanium rotary instruments. Int Endod J. 2012; 45:1148–1155.
Article30. D'Antò V, Rongo R, Ametrano G, Spagnuolo G, Manzo P, Martina R, et al. Evaluation of surface roughness of orthodontic wires by means of atomic force microscopy. Angle Orthod. 2012; 82:922–928.31. Rongo R, Ametrano G, Gloria A, Spagnuolo G, Galeotti A, Paduano S, et al. Effects of intraoral aging on surface properties of coated nickel-titanium archwires. Angle Orthod. 2014; 84:665–672.
Article32. Mandrich L, Cerreta M, Manco G. An engineered version of human PON2 opens the way to understand the role of its post-translational modifications in modulating catalytic activity. PLoS One. 2015; 10:e0144579.
Article33. Guillemot F, Prima F, Tokarev VN, Belin C, Porté-Durrieu MC, Gloriant T, et al. Ultraviolet laser surface treatment for biomedical applications of β titanium alloys: morphological and structural characterization. Appl Phys, A Mater Sci Process. 2003; 77:899–904.
Article34. Gallardo-Moreno AM, Pacha-Olivenza MA, Fernández-Calderón MC, Pérez-Giraldo C, Bruque JM, González-Martín ML. Bactericidal behaviour of Ti6Al4V surfaces after exposure to UV-C light. Biomaterials. 2010; 31:5159–5168.
Article35. Fox SC, Moriarty JD, Kusy RP. The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol. 1990; 61:485–490.
Article36. Mengel R, Buns CE, Mengel C, Flores-de-Jacoby L. An in vitro study of the treatment of implant surfaces with different instruments. Int J Oral Maxillofac Implants. 1998; 13:91–96.37. Hallmon WW, Waldrop TC, Meffert RM, Wade BW. A comparative study of the effects of metallic, nonmetallic, and sonic instrumentation on titanium abutment surfaces. Int J Oral Maxillofac Implants. 1996; 11:96–100.
Article38. Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants. 1996; 11:169–178.39. Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res. 2012; 23:643–658.
Article40. Bennani V, Hwang L, Tawse-Smith A, Dias GJ, Cannon RD. Effect of air-polishing on titanium surfaces, biofilm removal, and biocompatibility: a pilot study. BioMed Res Int. 2015; 2015:491047.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of biofilm on titanium and zirconia surfaces: in vivo study
- Effects of Biofilm Formation on The Antimicrobial Susceptibility of Staphylococcus aureus
- Adherence and Biofilm Formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on Spinal Implant
- Prevention of Biofilm Infections
- Stability and Efficacy of Titanium in Osteointegration in a Rabbit Model