Korean Lepr Bull.
2018 Dec;51(1):13-21. 10.33161/klb.2018.51.1.13.
Seropositivity of Hepatitis C Virus among Persons affected Leprosy in Korea
- Affiliations
-
- 1Institute for Leprosy Research, Korean Hansen Welfare Association, Korea. dr_jpkim@hotmail.com
- KMID: 2429659
- DOI: http://doi.org/10.33161/klb.2018.51.1.13
Abstract
- BACKGROUND
Leprosy is a contagious chronic granulomatous disease and is a disease that is associated with defects in cellular immunity. A high prevalence of hepatitis C virus infection in leprosy patients has been reported in several African countries, Yemen, Brazil and Japan. In Korea, it's seropositivity was reported as 8.33%(2001), 39.3%(2002), 35.1%(2009) and 16.0%(2009) on Korean Leprosy Bulletin.
OBJECTIVE
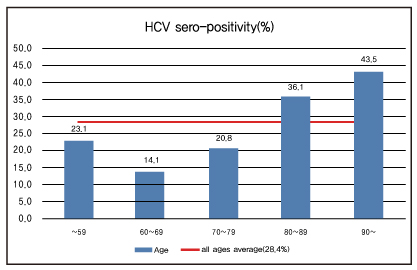
In the meantime, the studies were limited to the subjects in a specific region, and the number of subjects was not enough, so it was not enough to evaluate the hepatitis C virus seropositivity of persons affected by leprosy in Korea. So this study was conducted to evaluate the it's seropositivity in settlement villages nationwide. METHOD: This study was conducted that the mobile team visited the resettlement villages nationwide from 2009 to 2017 and conducted on persons affected by leprosy and residents residing in resettlement village. Obtained serums were assayed by the ADVIA Centaur HVC(IgG antibodies to Hepatitis C Virus) reagent using a Siemens ADVIA Centaur CP instrument. The results of persons affected by leprosy and residents were compared, and the difference of seropositivity among the groups(male and female, multibacillary and paucibacillary, locations of resettlement villages) was evaluated. RESULT: The results of hepatitis C virus antibody positivity of 1669 persons affected by leprosy subjects and 185 residents of resettlement villages were 28.46% in persons affected by leprosy and 6.49% in residents(Pearson's Chi-Square test, P = 0.00). In persons affected by leprosy, that were 31.99%(male) and 26.84%(female)(Pearson's Chi-Square test, P = 0.06) and were 29.97%(multibacillary) and 25.36%(paucibacillary)(Pearson's Chi-Square test, P = 0.05). That of Seoul(48.28%), Busan(43.78%) and Chungbuk Province(35.94%) were highly positive and that of Gangwon Province(20.34%) was lowly positive(Fisher's Exact test P = 0.002).
CONCLUSION
In this study, we found that hepatitis C virus antibody positivity rate was high in persons affected by leprosy in Korea. In order to explain the high positive rate, further studies will be needed. Also, through various approaches including assessment of HCV RNA to the subjects who were judged to be positive for antibody test in the future, a comprehensive evaluation of hepatitis C and its countermeasures are needed.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Management Outcome and Expansion Strategy for Treatment of Hepatitis C Virus Infection among Persons Affected with Leprosy
Min-Woo Lee, Hyung-Cheol Park
Korean Lepr Bull. 2019;52(1):51-54. doi: 10.33161/klb.2019.52.1.51.
Reference
-
1. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989; 244:359–362.2. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014; 61 1 Suppl:S45–S57.
Article3. Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013; 10:553–562.
Article4. Machado PR, Johnson WD, Glesby MJ. The role of human T cell lymphotrophic virus type 1, hepatitis B virus and hepatitis C virus coinfections in leprosy. Mem Inst Oswaldo Cruz. 2012; 107 Suppl 1:43–48.
Article5. Kim JP, Kim YS, Park TB, Ko YH. Status of Serum Hepatitis C Virus Antibodies in Hansen\'s Disease. Korean Lepr Bull. 2001; 34(2):81–89.6. Woo MJ, Kim SW, Hah YM, Bang YJ, Lee JY. Seroprevalence Study of Hepatitis C in Leprosy Patients. Korean Lepr Bull. 2002; 35(2):49–62.7. Kim JP, Lee RH. Status of Hepatitis C in Hansen\'s Disease. Korean Lepr Bull. 2009; 42(1):55–66.8. Choi SJ, Kim JW, Kim JP. Status of Hepatitis C of Hansen\'s Disease of Jeonbuk Province. Korean Lepr Bull. 2013; 46(1):63–72.9. Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, et al. An assay for circulation antidodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989; 244:362–364.10. Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut. 2012; 61 Suppl 1:i36–i46.11. Alter MJ. HCV routes of transmission: what goes around comes around. Semin Liver Dis. 2011; 31:340–346.
Article12. Kim do Y, Kim IH, Jeong SH, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int. 2013; 33:586–594.
Article13. Oh DJ, Park YM, Seo YI, Lee JS, Lee JY. Prevalence of hepatitis C virus infections and distribution of hepatitis C virus genotypes among Korean blood donors. Ann Lab Med. 2012; 32:210–215.
Article14. Kim MJ, Park Q, Min HK, Kim HO. Residual risk of trans- fusion-transmitted infection with human immunodeficiency virus, hepatitis C virus, and hepatitis B virus in Korea from 2000 through 2010. BMC Infect Dis. 2012; 12:160.
Article15. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of hepatitis C. Clin Mol Hepatol. 2014; 20:89–136.16. Lee SS, Jeong SH, Jang ES, Kim YS, Lee YJ, Jung EU, et al. A prospective cohort study on the outcomes of hepatitis C virus-related cirrhosis in South Korea. J Gastroenterol Hepatol. 2015; 30(8):1281–1287.17. Lee SS, Byoun YS, Jeong SH, Kim YM, Gil H, Min BY, et al. Type and cause of liver disease in Korea: single-center experience, 2005-2010. Clin Mol Hepatol. 2012; 18:309–315.
Article18. Rosa H, Martins R, Vanderborght B. Short report: association between leprosy and hepatitis C infections: a survey in a region Central Brazil. Am J Trop Med Hyg. 1996; 55:22–23.19. de Moraes Braga AC, Reason IJ, Maluf EC, Vieira ER. Leprosy and confinement due to leprosy show high association with hepatitis C in Southern Brazil. Acta tropica. 2006; 97:88–93.
Article20. Shiogama K, Teramoto H, Morita Y, Mizutani Y, Shimomura R, Inada K, Kamahora T, Makino M, Tsutsumi Y. Hepatitis C virus infection in a Japanese leprosy sanatorium for the past 67 years. J Med Virol. 2010; 82:556–561.
Article21. Ramos JM, Costa e Silva AM, Martins RM, Souto FJ. Prevalence of hepatitis B and C virus infection among leprosy patients in a leprosy-endemic region of Central Brazil. Mem Inst Oswaldo Cruz. 2011; 106:632–634.
Article22. Frommel D, Tekle-Haimanot R, Berhe N, Aussel L, Verdier M, Preux PM, Denis F. A survey of antibodies to hepatitis C virus in Ethiopia. Am J Trop Med Hyg. 1993; 49:435–439.
Article23. Denis F, Aussel L, Ranger S, Martin P, Itoua-N\'Gaporo A, Frommel D, Teckle-Haimanot RT, et al. Prevalence of antibodies to hepatitis C virus among patients with leprosy in several African countries and the Yemen. J Med Virol. 1994; 43:1–4.
Article24. Egawa K, Yukawa T, Arakawa S, Tanaka T, Tsuda F, Okamoto H, Miyakawa Y, et al. Hepatitis C virus antibody, viral RNA and genotypes in leprous patients in Japan. J Hepatol. 1996; 24:397–402.
Article25. Rego VP, Machado PR, Martins I, Trindade R, Paraná R. Type 1 reaction in leprosy: characteristics and association with hepatitis B and C viruses. Rev Soc Bras Med Trop. 2007; 40:546–549.26. Kim KA, Jeong SH, Jang ES, Kim YS, Lee YJ, Jung EU, et al. Geographic differences in the epidemiological features of HCV infection in Korea. Clin Mol Hepatol. 2014; 20:361–367.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Management Outcome and Expansion Strategy for Treatment of Hepatitis C Virus Infection among Persons Affected with Leprosy
- Status of Hepatitis C in Hansen's Disease
- Status of Hepatitis C of Hansen's Disease of Jeonbuk Province
- Prevention of Viral Hepatitis and Vaccination
- The Study of Status about Hearing Loss in Person Affected Leprosy