Ann Lab Med.
2018 Nov;38(6):512-517. 10.3343/alm.2018.38.6.512.
An Automated Draft Report Generator for Peripheral Blood Smear Examinations Based on Complete Blood Count Parameters
- Affiliations
-
- 1Department of Laboratory Medicine, Korea University College of Medicine, Seoul, Korea. labmd@korea.ac.kr
- 2Department of Laboratory Medicine, Inha University School of Medicine, Incheon, Korea.
- KMID: 2429115
- DOI: http://doi.org/10.3343/alm.2018.38.6.512
Abstract
- BACKGROUND
Complete blood count (CBC) results play an important role in peripheral blood smear (PBS) examinations. Many descriptions in PBS reports may simply be translated from CBC parameters. We developed a computer program that automatically generates a PBS draft report based on CBC parameters and age- and sex-matched reference ranges.
METHODS
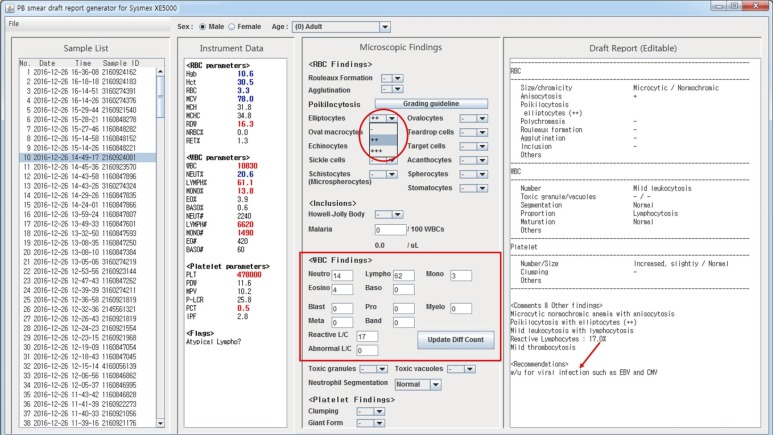
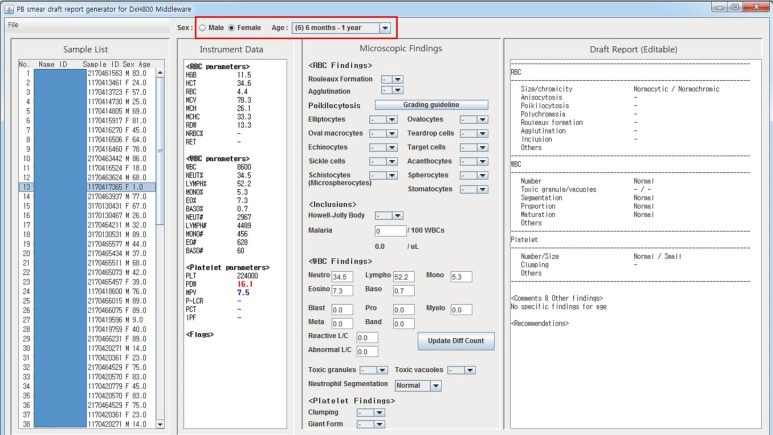
The Java programming language was used to develop a computer program that supports a graphical user interface. Four hematology analyzers from three different laboratories were tested: Sysmex XE-5000 (Sysmex, Kobe, Japan), Sysmex XN-9000 (Sysmex), DxH800 (Beckman Coulter, Brea, CA, USA), and ADVIA 2120i (Siemens Healthcare Diagnostics, Eschborn, Germany). Input data files containing 862 CBC results were generated from hematology analyzers, middlewares, or laboratory information systems. The draft reports were compared with the content of input data files.
RESULTS
We developed a computer program that reads CBC results from a data file and automatically writes a draft PBS report. Age- and sex-matched reference ranges can be automatically applied. After examining PBS, users can modify the draft report based on microscopic findings. Recommendations such as suggestions for further evaluations are also provided based on morphological findings, and they can be modified by users. The program was compatible with all four hematology analyzers tested.
CONCLUSIONS
Our program is expected to reduce the time required to manually incorporate CBC results into PBS reports. Systematic inclusion of CBC results could help improve the reliability and sensitivity of PBS examinations.
MeSH Terms
Figure
Reference
-
1. Bain BJ. Diagnosis from the blood smear. N Engl J Med. 2005; 353:498–507. PMID: 16079373.2. McPherson RA, Pincus MR. Henry's clinical diagnosis and management by laboratory methods. 23rd ed. Missouri, MO: Elsevier;2017. p. 519–521.3. Palmer L, Briggs C, McFadden S, Zini G, Burthem J, Rozenberg G, et al. ICSH recommendations for the standardization of nomenclature and grading of peripheral blood cell morphological features. Int J Lab Hematol. 2015; 37:287–303. PMID: 25728865.4. McFarlane A, Aslan B, Raby A, Bourner G, Padmore R. Critical values in hematology. Int J Lab Hematol. 2015; 37:36–43.5. Vaughan J, Alli N, Mannaru K, Sedick Q. Refining peripheral blood smear review rules for neonatal inpatients in a South African academic laboratory. Int J Lab Hematol. 2016; 38:347–351. PMID: 27087063.6. Proytcheva MA. Issues in neonatal cellular analysis. Am J Clin Pathol. 2009; 131:560–573. PMID: 19289592.7. Mayo Medical Laboratories. Pediatric test reference values. Updated on Jun 2018. http://www.mayomedicallaboratories.com/test-info/pediatric/refvalues/reference.php.8. Murari M, Pandey R. A synoptic reporting system for bone marrow aspiration and core biopsy specimens. Arch Pathol Lab Med. 2006; 130:1825–1829. PMID: 17149957.9. Mohanty SK, Piccoli AL, Devine LJ, Patel AA, William GC, Winters SB, et al. Synoptic tool for reporting of hematological and lymphoid neoplasms based on World Health Organization classification and College of American Pathologists checklist. BMC Cancer. 2007; 7:144. PMID: 17672904.10. Jaso J, Nguyen A, Nguyen AN. A synoptic reporting system for peripheral blood smear interpretation. Am J Clin Pathol. 2011; 135:358–364. PMID: 21350088.11. McFadden SL, Machin SJ, Simson E. The International Consensus Group for Hematology Review: suggested criteria for action following automated CBC and WBC differential analysis. Lab Hematol. 2005; 11:83–90. PMID: 16024331.12. Nathan D, Oski F. Nathan and Oski's hematology of infancy and childhood. 7th ed. Philadelphia, PA: Saunders/Elsevier;2009. p. 1841–1848.13. Park SH, Park CJ, Lee BR, Kim MJ, Han MY, Cho YU, et al. Establishment of age-and gender-specific reference ranges for 36 routine and 57 cell population data items in a new automated blood cell analyzer, Sysmex XN-2000. Ann Lab Med. 2016; 36:244–249. PMID: 26915613.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Purpose and Criteria for Blood Smear Scan, Blood Smear Examination, and Blood Smear Review
- A Case of Spuriously Decreased White Blood Cell Count on an Automated Sysmex XN Hematology Analyzer: The Difference Between the WNR and WDF Channels
- Misidentification of Candida parapsilosis as Large Platelets in an Automated Blood Analyzer
- An interpretation on abnormal finding of CBC
- A Case of Pseudoleukopenia due to Cold-Induced Leukocyte Agglutination