Yonsei Med J.
2005 Dec;46(6):799-805.
A Pilot Study of Trans-Arterial Injection of 166Holmium-Chitosan Complex for Treatment of Small Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Diagnostic Radiology, Yonsei University College of Medicine, Seoul, Korea.
- 4Laboratory of Nuclear Medicine, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
Abstract
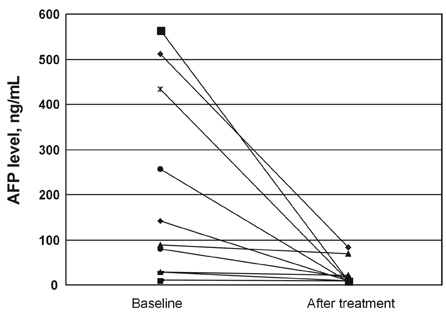
- Percutaneous approaches, such as percutaneous ethanol injection and radiofrequency ablation, have been most widely used for hepatocellular carcinoma patients who were not eligible for surgery. New technologies to improve the efficacy are currently needed. 166Holmium is a neutron activated radionuclide, and has several beneficial radiophysical characteristics for internal radiation therapy. 166Holmium-Chitosan complex, in which chitosan is chelated with 166Holmium, was developed as a radiopharmaceutical for cancer therapy. We have conducted a pilot study to evaluate the clinical efficacy of transarterial administration of 166Holmium-Chitosan complex in patients with a single and small (< 3 cm) hepatocellular carcinoma. 166Holmium-Chitosan complex, at a dose of 20 mCi per cm of tumor mass-diameter, was administered through the artery that directly fed the tumor. Twelve patients were treated with a median follow-up duration of 26 (range: 12-61) months. The tumor diameter ranged between 1.5 and 2.5 cm. Ten patients (83%) had complete response and two (17%) had partial response. The median complete response duration was not reached. The median AFP level declined from 83.8 to 8.3 ng/mL within 2 months after treatment. No grade III/IV toxicity was observed. Grade I and II toxicities were observed in four patients (2 abdominal pain, 1 fever, and 1 AST/ALT elevation). No toxic death occurred. This preliminary study shows a promising and durable complete response rate with an acceptable safety profile. Further studies with greater accrual of patients are warranted.
Keyword
MeSH Terms
-
alpha-Fetoproteins/metabolism
Tomography, X-Ray Computed
Radiopharmaceuticals/administration & dosage/*therapeutic use
Pilot Projects
Middle Aged
Male
Liver Neoplasms/pathology/radiography/*radiotherapy
Injections, Intra-Arterial
Humans
Female
Chitosan/administration & dosage/*therapeutic use
Carcinoma, Hepatocellular/pathology/radiography/*radiotherapy
Aged
Adult
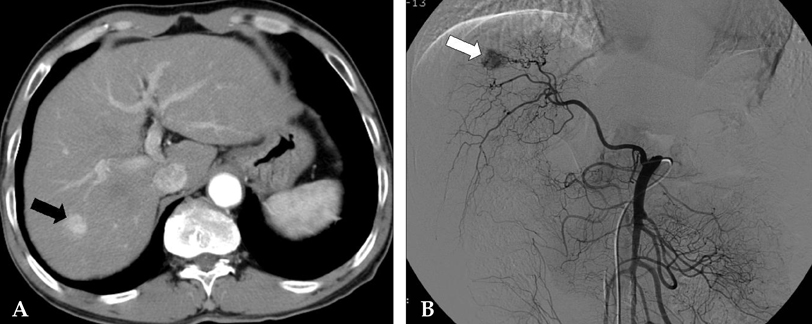
Figure
Reference
-
1. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003. 362:1907–1917.2. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radio-frequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004. 127:1714–1723.3. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–661.4. Nijsen JF, Zonnenberg BA, Woittiez JR, Rook DW, Swildens-van Woudenberg IA, van Rijk PP, et al. Holmium-166 poly lactic acid microspheres applicable for intra-arterial radionuclide therapy of hepatic malignancies: effects of preparation and neutron activation techniques. Eur J Nucl Med. 1999. 26:699–704.5. Mumper RJ, Ryo UY, Jay M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres: a potential agent for the internal radiation therapy of hepatic tumors. J Nucl Med. 1991. 32:2139–2143.6. Nijsen F, Rook D, Brandt C, Meijer R, Dullens H, Zonnenberg B, et al. Targeting of liver tumour in rats by selective delivery of holmium-166 loaded microspheres: a biodistribution study. Eur J Nucl Med. 2001. 28:743–749.7. Muzzarelli R, Baldassarre V, Conti F, Ferrara P, Biagini G, Gazzanelli G, et al. Biological activity of chitosan: ultrastructural study. Biomaterials. 1988. 9:247–252.8. Hirano S, Noishiki Y. The blood compatibility of chitosan and N-acylchitosans. J Biomed Mater Res. 1985. 19:413–417.9. Rao SB, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and hemostatic potential. J Biomed Mater Res. 1997. 34:21–28.10. Sohn JH, Lee JT, Lee JD, Chung HC, Kim JH, Yoo NC, et al. Transarterial injection of Holmium-166-Chitosan complex (Hol-166) in the treatment of single and large hepatocellular carcinoma: A novel therapeutic modality. ASCO Annual Meeting Proceedings. 2004. 22:4023.11. Nelson WR, Hirayam H, Rogers DWO. The EGS 4 code system. 1985. Standard Linear Accelerator Center, SLAC;256.12. Lau WY, Leung WT, Ho S, Leung NW, Chan M, Lin J, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994. 70:994–999.13. Salem R, Lewandowski R, Roberts C, Goin J, Thurston K, Abouljoud M, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol. 2004. 15:335–345.14. Leung TW, Lau WY, Ho SK, Ward SC, Chow JH, Chan MS, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995. 33:919–924.15. Kim JK, Han KH, Lee JT. The long term therapeutic efficacy and the safety of percutaneous holmium injection for the treatment of small hepatocellular carcinoma. J Hepatol. 2003. 38:Suppl 2. 6.16. Suzuki YS, Momose Y, Higashi N, Shigematsu A, Park KB, Kim YM, et al. Biodistribution and kinetics of holmium-166-chitosan complex (DW-166HC) in rats and mice. J Nucl Med. 1998. 39:2161–2166.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intratumoral Injection of 166Holmium-chitosan Complex to SmallRenal Cell Carcinoma: Preliminary Results
- Effects of 166Holmium and 166Holmium-chitosan Complex(166Ho-CHICO) on Normal Brain of Rats
- Experimental and Clinical Studies on the Intraarterial Injection of Holmium-166 Chitosan Complex in the Treatment of Hepatocellular Carcinoma
- Radiation Synovectomy by 166Holmium-Chitosan complex in Collagenase Induced Arthritis of the Knee in the Rabbit
- Interventional Treatment of Hepatocellular Carcinoma; Transcatheter Arterial Chemoembolization and Percutaneous Ethanol Injection