Yonsei Med J.
2005 Dec;46(6):750-758.
Comparison of Effect of Treatment with Etidronate and Alendronate on Lumbar Bone Mineral Density in Elderly Women with Osteoporosis
- Affiliations
-
- 1Department of Sports Medicine, Keio University School of Medicine, Tokyo, Japan.
- 2Department of Neurology, Mitate Hospital, Fukuoka, Japan.
- 3Department of Orthopaedic Surgery, Keiyu Orthopaedic Hospital, Gunma, Japan.
Abstract
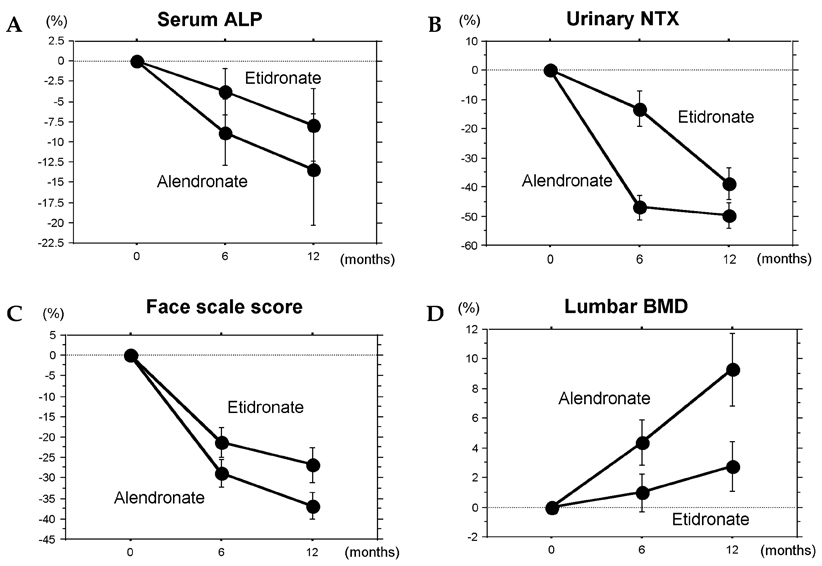
- The purpose of this open-labeled prospective study was to compare the treatment effects of cyclical etidronate and alendronate on the lumbar bone mineral density (BMD), bone resorption, and back pain in elderly women with osteoporosis. Fifty postmenopausal women with osteoporosis, age ranging from 55 to 86 years (mean: 70.7 years), were randomly divided into two groups with 25 patients in each group: the cyclical etidronate group (etidronate 200 mg daily for 2 weeks every 3 months) and the alendronate group (5 mg daily). The BMD of the lumbar spine (L1-L4) measured by DXA, the urinary cross-linked N-terminal telopeptides of type I collagen (NTX) level measured by the enzyme-linked immunosorbent assay, and back pain evaluated by the face scale score were assessed at baseline, 6 months, and 12 months. There were no significant differences in baseline characteristics including age, body mass index, years since menopause, lumbar BMD, urinary NTX level, and face scale score between the two treatment groups. Etidronate treatment sustained the lumbar BMD following a reduction in the urinary NTX level and improved back pain, while alendronate treatment reduced the urinary NTX level more significantly, resulting in an increase in the lumbar BMD, and similarly improved back pain. No serious adverse events were observed in either group. This study confirmed that alendronate treatment had a greater efficacy than etidronate treatment in increasing the lumbar BMD through the reduction of bone resorption in elderly women with osteoporosis.
MeSH Terms
-
Spinal Fractures/prevention & control/radiography
Osteoporosis, Postmenopausal/*drug therapy
Middle Aged
Lumbar Vertebrae/*drug effects
Humans
Female
Etidronic Acid/adverse effects/*therapeutic use
Bone Density Conservation Agents/adverse effects/*therapeutic use
Bone Density/*drug effects
Biological Markers/blood/urine
Back Pain/drug therapy
Alendronate/adverse effects/*therapeutic use
Aged, 80 and over
Aged
Figure
Reference
-
1. Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, et al. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. Osteoporos Int. 1999. 10:183–192.2. Kushida K, Shiraki M, Nakamura T, Kishimoto H, Morii H, Yamamoto K, et al. The efficacy of alendronate in reducing the risk for vertebral fracture in Japanese patients with osteoporosis: a randomized, double-blind, active-controlled, double-dummy trial. Current Therapeutic Research. 2002. 63(9):606–620.3. Fujita T, Orimo H, Inoue T, Kaneda K, Sakurai M, Morita R, et al. Double-blind multicenter comparative study with alfacalcidol of etidronate disodium (EHDP) in involutional osteoporosis (in Japanese). Clin Eval. 1993. 21:261–302.4. Rogers MJ, Frith JC, Luckman SP, Coxon FR, Benford HL, Monkkonen J, et al. Molecular mechanism of action of bisphosphonate. Bone. 1999. 24:5 Suppl. 73S–79S.5. Cranny A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C. Meta-analyses of therapies for postmenopausal osteoporosis. IX Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002. 23:570–578.6. Nevitt MC, Thompson DE, Black DM, Rubin SR, Ensrud K, Yates AJ, et al. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Arch Intern Med. 2000. 160:77–85.7. Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, et al. Diagnostic criteria of primary osteoporosis. J Bone Miner Metab. 1998. 16:139–150.8. Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001. 19:331–337.9. Lorish CD, Maisiak R. The face scale: a brief, nonverbal method for assessing patient mood. Arthritis Rheum. 1986. 29:906–909.10. Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994. 79:1693–1700.11. Greenspan SL, Parker RA, Ferguson L, Rosen NH, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res. 1998. 13:1431–1438.12. Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000. 85:3109–3115.13. Orimo H, Shiraki M, Hayashi Y, Hoshino T, Onaya T, Mayazaki S, et al. Effects of 1α-hydroxyvitamin D3 on lumbar bone mineral density and vertebral fractures in patients with osteoporosis. Calcif Tissue Int. 1994. 54:370–376.14. Agarwala S, Sule A, Pai BU, Joshi VR. Alendronate in the treatment of avascular necrosis of the hip. Rheumatology (Oxford). 2002. 41:346–347.15. Gangji V, Appelboom T. Analgesic effect of intravenous pamidronate on chronic back pain due to osteoporotic vertebral fractures. Clin Rheumatol. 1999. 18:266–267.16. Glover D, Lipton A, Keller A, Miller AA, Browing S, Fram RJ, et al. Intravenous pamidronate disodium treatment of bone metastases in patients with breast cancer. A dose-seeking study. Cancer. 1994. 74:2949–2955.17. Iwamoto J, Takeda T, Ichimura S. Transient relief of metastatic cancer bone pain by oral administration of etidronate. J Bone Miner Metab. 2002. 20:228–234.18. Hortobagyi GN, Terhiault RL, Porter L, Blayney D, Lipton A, Sinoff C, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med. 1996. 335:1785–1791.19. Siris E, Chines AA, Altman RD, Brown JP, Johnston CC Jr, Lang R, et al. Risedronate in the treatment of Paget's disease of bone: an open label, multicenter study. J Bone Miner Res. 1998. 13:1032–1038.20. Nishikaku F, Nakayama T, Nakatsuka M. Analgesic property of bisphosphonate, etidronate in animal models. Jpn Pharmacol Ther. 1998. 26:457–463. (in Japanese).21. Tanaka T, Nakayama T, Katsumata T. Etidronate, therapeutic agent for osteoporosis possessing analgesic effect. Bio Clinica. 2001. 16:57–61. (in Japanese).22. Pappagallo M, Breuer B, Schneider A, Sperber K. Treatment of chronic mechanical spinal pain with intravenous pamidronate: a review of medical records. J Pain Symptom Manage. 2003. 26:678–683.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction: Paper “Comparison of Effect of Treatment with Etidronate and Alendronate on Lumbar Bone Mineral Density in Elderly Women with Osteoporosis†by Iwamoto J, et al. [Yonsei Med J 2005;46(6):750-758]
- Combined Treatment with Vitamin K2 and Bisphosphonate in Postmenopausal Women with Osteoporosis
- Comparison of Effects of Alendronate and Raloxifene on Lumbar Bone Mineral Density, Bone Turnover, and Lipid Metabolism in Elderly Women with Osteoporosis
- Change of Bone Mineral Density after One-year Alendronate Treatment in Premenopausal Women with Low Bone Density
- Combination Therapy of Raloxifene and Alendronate for Treatment of Osteoporosis in Elderly Women