Yonsei Med J.
2019 Jan;60(1):30-37. 10.3349/ymj.2019.60.1.30.
Correlation of UGT2B7 Polymorphism with Cardiotoxicity in Breast Cancer Patients Undergoing Epirubicin/Cyclophosphamide-Docetaxel Adjuvant Chemotherapy
- Affiliations
-
- 1Department of Thyroid and Breast Surgery, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. zhangxiaoyee@163.com
- KMID: 2428275
- DOI: http://doi.org/10.3349/ymj.2019.60.1.30
Abstract
- PURPOSE
The present study aimed to investigate correlations between uridine glucuronosyltransferase 2B7 (UGT2B7) -161 single nucleotide polymorphism C to T (C>T) and the occurrence of cardiotoxicity in Chinese breast cancer (BC) patients undergoing epirubicin/cyclophosphamide-docetaxel (EC-D) adjuvant chemotherapy.
MATERIALS AND METHODS
427 BC patients who had underwent surgery were consecutively enrolled in this prospective cohort study. All patients were scheduled to receive EC-D adjuvant chemotherapy regimen, and they were divided into UGT2B7 -161 CC (n=141), UGT2B7 -161 CT (n=196), and UGT2B7 -161 TT (n=90) groups according to their genotypes. Polymerase chain reaction was performed for determination of UGT2B7 -161 genotypes. Cardiotoxicity was defined as an absolute decline in left ventricular ejection fraction (LVEF) of at least 10% points from baseline to a value less than 53%, heart failure, acute coronary artery syndrome, or fatal arrhythmia.
RESULTS
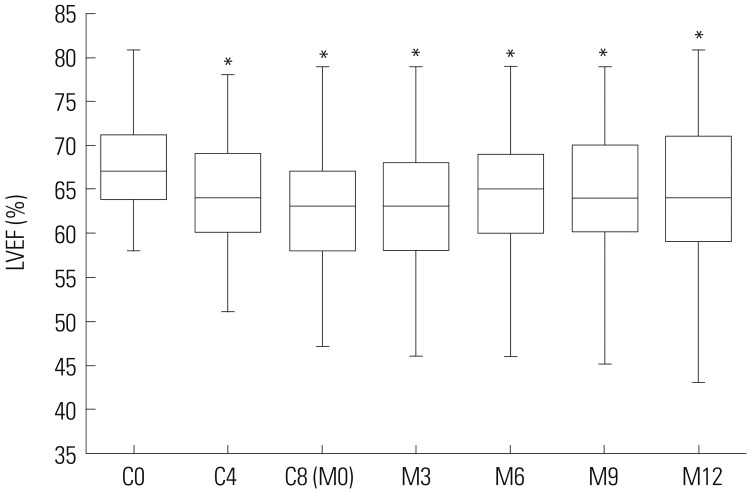
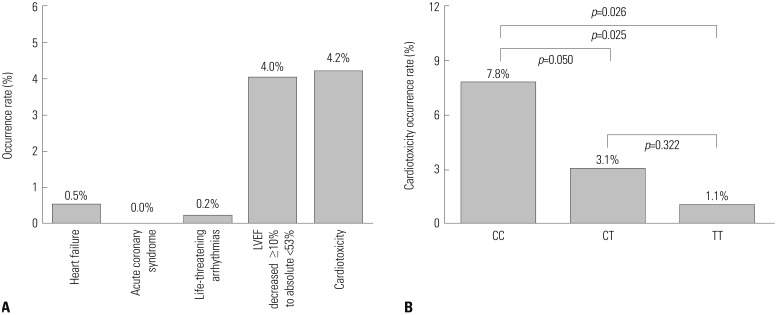
LVEF values were lower at cycle (C) 4, C8, 3 months after chemotherapy (M3), M6, M9, and M12 compared to C0 (all p < 0.001), in BC patients undergoing EC-D adjuvant chemotherapy. Cardiotoxicity was recorded for 4.2% of the overall population and was lowest in the UGT2B7 -161 TT group (1.1%), compared to UGT2B7 -161 CT (3.1%) and UGT2B7 -161 CC (7.8%) group (p=0.026). Multivariate logistic regression revealed that UGT2B7 -161 T allele could independently predict a low occurrence of cardiotoxicity in BC patients undergoing EC-D adjuvant chemotherapy (p=0.004).
CONCLUSION
A UGT2B7 -161 T allele serves as a potential biomarker for predicting a low occurrence of cardiotoxicity in BC patients undergoing EC-D adjuvant chemotherapy.
Keyword
MeSH Terms
-
Alleles
Arrhythmias, Cardiac
Asian Continental Ancestry Group
Breast Neoplasms*
Breast*
Cardiotoxicity*
Chemotherapy, Adjuvant*
Cohort Studies
Coronary Vessels
Drug Therapy
Genotype
Glucuronosyltransferase
Heart Failure
Humans
Logistic Models
Polymerase Chain Reaction
Polymorphism, Single Nucleotide
Prospective Studies
Stroke Volume
Uridine
Glucuronosyltransferase
Uridine
Figure
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID: 25651787.
Article2. Jiang L, Jing C, Kong X, Li X, Ma T, Huo Q, et al. Comparison of adjuvant ED and EC-D regimens in operable breast invasive ductal carcinoma. Oncol Lett. 2016; 12:1448–1454. PMID: 27446451.
Article3. Ejlertsen B, Tuxen MK, Jakobsen EH, Jensen MB, Knoop AS, Højris I, et al. Adjuvant cyclophosphamide and docetaxel with or without epirubicin for early TOP2A-normal breast cancer: DBCG 07-READ, an open-label, phase III, randomized trial. J Clin Oncol. 2017; 35:2639–2646. PMID: 28661759.
Article4. Domercant J, Polin N, Jahangir E. Cardio-oncology: a focused review of anthracycline-, human epidermal growth factor receptor 2 inhibitor-, and radiation-induced cardiotoxicity and management. Ochsner J. 2016; 16:250–256. PMID: 27660573.5. Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014; 63:809–816. PMID: 24291281.
Article6. Appel JM, Sogaard P, Mortensen CE, Skagen K, Nielsen DL. Tissue-Doppler assessment of cardiac left ventricular function during short-term adjuvant epirubicin therapy for breast cancer. J Am Soc Echocardiogr. 2011; 24:200–206. PMID: 21227647.
Article7. Parmar S, Stingl JC, Huber-Wechselberger A, Kainz A, Renner W, Langsenlehner U, et al. Impact of UGT2B7 His268Tyr polymorphism on the outcome of adjuvant epirubicin treatment in breast cancer. Breast Cancer Res. 2011; 13:R57. PMID: 21658222.
Article8. Sawyer MB, Pituskin E, Damaraju S, Bies RR, Vos LJ, Prado CM, et al. A uridine glucuronosyltransferase 2B7 polymorphism predicts epirubicin clearance and outcomes in early-stage breast cancer. Clin Breast Cancer. 2016; 16:139–144. PMID: 26452313.
Article9. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28:1–39. PMID: 25559473.
Article10. Kitayama H, Kondo T, Sugiyama J, Kurimoto K, Nishino Y, Kawada M, et al. High-sensitive troponin T assay can predict anthracycline-and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer. 2017; 24:774–782. PMID: 28434150.11. Stewart GP, Bagley RL, Froemling RA. Evaluation of reversible hydrocolloid impression material in a wet field. J Prosthet Dent. 1984; 51:797–800. PMID: 6376782.
Article12. Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012; 104:1293–1305. PMID: 22949432.
Article13. Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol. 2001; 19:2746–2753. PMID: 11352968.
Article14. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011; 365:1273–1283. PMID: 21991949.
Article15. Jain KK, Casper ES, Geller NL, Hakes TB, Kaufman RJ, Currie V, et al. A prospective randomized comparison of epirubicin and doxorubicin in patients with advanced breast cancer. J Clin Oncol. 1985; 3:818–826. PMID: 3859587.
Article16. Cottin Y, Touzery C, Dalloz F, Coudert B, Toubeau M, Riedinger A, et al. Comparison of epirubicin and doxorubicin cardiotoxicity induced by low doses: evolution of the diastolic and systolic parameters studied by radionuclide angiography. Clin Cardiol. 1998; 21:665–670. PMID: 9755384.17. Innocenti F, Iyer L, Ramírez J, Green MD, Ratain MJ. Epirubicin glucuronidation is catalyzed by human UDP-glucuronosyltransferase 2B7. Drug Metab Dispos. 2001; 29:686–692. PMID: 11302935.18. Areepium N, Panomvana D, Rungwanonchai P, Sathaporn S, Voravud N. Effects of CYP2D6 and UGT2B7 polymorphisms on pharmacokinetics of tamoxifen in Thai breast cancer patients. Breast Cancer (Dove Med Press). 2013; 5:73–78. PMID: 24648760.19. Bastami S, Gupta A, Zackrisson AL, Ahlner J, Osman A, Uppugunduri S. Influence of UGT2B7, OPRM1 and ABCB1 gene polymorphisms on postoperative morphine consumption. Basic Clin Pharmacol Toxicol. 2014; 115:423–431. PMID: 24703092.20. National Center for Biotechnology Information. Reference SNP (refSNP) cluster report: rs7668258. accessed on 2018 April 2. Available at: https://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=7668258.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MicroRNA-130a Increases and Predicts Cardiotoxicity during Adjuvant Chemotherapy in Human Epidermal Growth Factor Receptor-2-Positive Breast Cancer
- Induction Chemotherapy for Breast Cancer: A case control study
- The Prognosis of breast Cancr with more than 10 Positive Nodes
- Incidence of Febrile Neutropenia in Korean Female Breast Cancer Patients Receiving Preoperative or Postoperative Doxorubicin/Cyclophosphamide Followed by Docetaxel Chemotherapy
- The evidence for adjuvant taxanes in early breast cancer