Korean J Sports Med.
2018 Dec;36(4):214-220. 10.5763/kjsm.2018.36.4.214.
Effect of Ultramarathon on the Anterior Pituitary and Thyroid Hormones
- Affiliations
-
- 1Department of Clinical Laboratory Science, Shinsung University, Dangjin, Korea.
- 2Department of Exercise Rehabilitation Welfare, Sungshin University, Seoul, Korea. kyj87@sungshin.ac.kr
- KMID: 2428005
- DOI: http://doi.org/10.5763/kjsm.2018.36.4.214
Abstract
- PURPOSE
The purpose of this research is to study changes in pituitary hormone in anterior lobe and thyroid hormone before, after, and during recovery time in severe 100 km ultramarathon.
METHODS
Healthy middle-aged runners (age, 52.0±4.8 years) participated in the test. Grade exercise test is done, and then blood is taken from those participants before and after completing 100 km ultramarathon at the intervals of 24 hours (1 day), 72 hours (3 days), and 120 hours (5 days) to analyze their luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyroid stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4), and free thyroxine (Free T4).
RESULTS
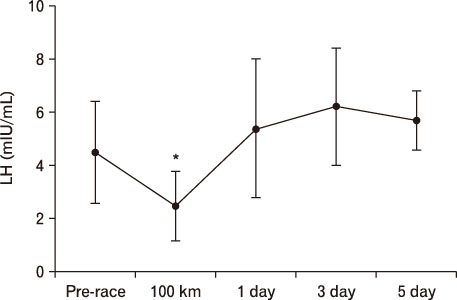
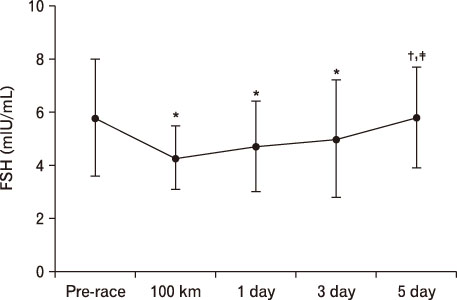
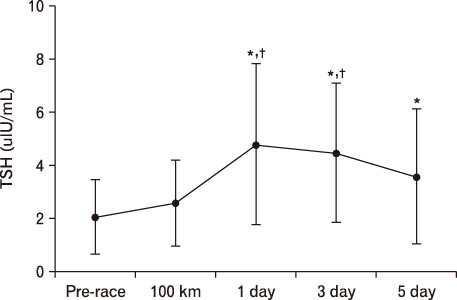
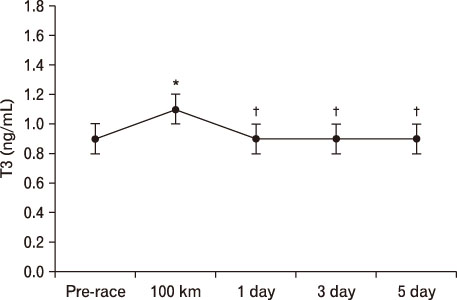
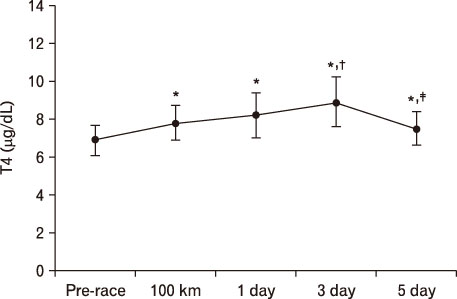
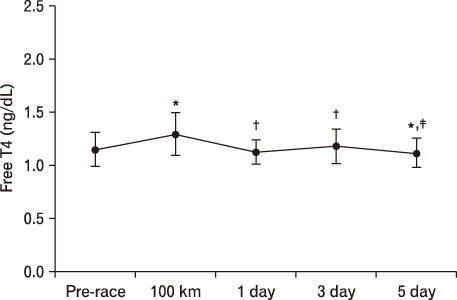
For LH, it decreased more significantly at 100 km than pre-race. However, after 1 day result increased more than that of 100 km. At 3 days, it was significantly higher than pre-race and 100 km, recovering at 5 days. In terms of FSH, it decreased at 100 km, 1 day, and 3 days more than pre-race but recovered at 5 days. TSH was higher at 1 day and 5 days compared to pre-race. T3 was only higher at 100 km than pre-race. T4 was higher till 5 days at 100 km than pre-race. Free T4 increased more significantly at 100 km than pre-race.
CONCLUSION
In terms of severe long distance running, LH and FSH which belong to hormone from anterior lobe as well as T3, T4, and Free T4 which belong to thyroid hormone showed their variation within the standard range. However, TSH showed abnormal increase from enhanced concentration of blood after marathon becoming hyper-activation even during the recovery period.
Keyword
MeSH Terms
Figure
Reference
-
1. Bluher M, Zimmer P. Metabolic and cardiovascular effects of physical activity, exercise and fitness in patients with type 2 diabetes. Dtsch Med Wochenschr. 2010; 135:930–934.2. Bobbert T, Mai K, Brechtel L, et al. Leptin and endocrine parameters in marathon runners. Int J Sports Med. 2012; 33:244–248.
Article3. Geyssant A, Geelen G, Denis C, et al. Plasma vasopressin, renin activity, and aldosterone: effect of exercise and training. Eur J Appl Physiol Occup Physiol. 1981; 46:21–30.
Article4. Galbo H. Integrated endocrine responses and exercise. In : DeGroot LJ, editor. Endocrinology. 3rd ed. Philadelphia (PA): W.B. Saunders;1995. p. 2692–2701.5. Ball D. Metabolic and endocrine response to exercise: sympathoadrenal integration with skeletal muscle. J Endocrinol. 2015; 224:R79–R95.
Article6. Melmed S, Park J, Hershman JM. Triiodothyronine induces a transferable factor which suppresses TSH secretion in cultured mouse thyrotropic tumor cells. Biochem Biophys Res Commun. 1981; 98:1022–1028.
Article7. Miller PB, Forstein DA, Styles S. Effect of short-term diet and exercise on hormone levels and menses in obese, infertile women. J Reprod Med. 2008; 53:315–319.8. Weiss EP, Villareal DT, Racette SB, et al. Caloric restriction but not exercise-induced reductions in fat mass decrease plasma triiodothyronine concentrations: a randomized controlled trial. Rejuvenation Res. 2008; 11:605–609.
Article9. Lehmann M, Dickhuth HH, Gendrisch G, et al. Trainingovertraining: a prospective, experimental study with experienced middle- and long-distance runners. Int J Sports Med. 1991; 12:444–452.10. Wheeler GD, Singh M, Pierce WD, Epling WF, Cumming DC. Endurance training decreases serum testosterone levels in men without change in luteinizing hormone pulsatile release. J Clin Endocrinol Metab. 1991; 72:422–425.
Article11. Scharnhorst V, Valkenburg J, Vosters C, Vader H. Influence of preanalytical factors on the immulite intact parathyroid hormone assay. Clin Chem. 2004; 50:974–975.
Article12. Tremblay MS, Copeland JL, Van Helder W. Effect of training status and exercise mode on endogenous steroid hormones in men. J Appl Physiol (1985). 2004; 96:531–539.
Article13. Tremblay MS, Copeland JL, Van Helder W. Influence of exercise duration on post-exercise steroid hormone responses in trained males. Eur J Appl Physiol. 2005; 94:505–513.
Article14. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974; 37:247–248.
Article15. Beutler J, Schmid E, Fischer S, Hurlimann S, Konrad C. Sudden cardiac death during a city marathon run. Anaesthesist. 2015; 64:451–455.16. Noakes T. Hyponatremia in distance runners: fluid and sodium balance during exercise. Curr Sports Med Rep. 2002; 1:197–207.17. Vlachopoulos C, Kardara D, Anastasakis A, et al. Arterial stiffness and wave reflections in marathon runners. Am J Hypertens. 2010; 23:974–979.
Article18. Shin KA, Park KD, Ahn J, Park Y, Kim YJ. Comparison of changes in biochemical markers for skeletal muscles, hepatic metabolism, and renal function after three types of long-distance running: observational study. Medicine (Baltimore). 2016; 95:e3657.19. Kim YJ, Shin YO, Lee JB, et al. The effects of running a 308 km ultra-marathon on cardiac markers. Eur J Sport Sci. 2014; 14:Suppl 1. S92–S97.
Article20. MacConnie SE, Barkan A, Lampman RM, Schork MA, Beitins IZ. Decreased hypothalamic gonadotropin-releasing hormone secretion in male marathon runners. N Engl J Med. 1986; 315:411–417.
Article21. Kuusi T, Kostiainen E, Vartiainen E, et al. Acute effects of marathon running on levels of serum lipoproteins and androgenic hormones in healthy males. Metabolism. 1984; 33:527–531.
Article22. McColl EM, Wheeler GD, Gomes P, Bhambhani Y, Cumming DC. The effects of acute exercise on pulsatile LH release in high-mileage male runners. Clin Endocrinol (Oxf). 1989; 31:617–621.
Article23. Kupchak BR, Kraemer WJ, Hoffman MD, Phinney SD, Volek JS. The impact of an ultramarathon on hormonal and biochemical parameters in men. Wilderness Environ Med. 2014; 25:278–288.
Article24. Vasankari TJ, Kujala UM, Heinonen OJ, Huhtaniemi IT. Effects of endurance training on hormonal responses to prolonged physical exercise in males. Acta Endocrinol (Copenh). 1993; 129:109–113.
Article25. Moore AW, Timmerman S, Brownlee KK, Rubin DA, Hackney AC. Strenuous, fatiguing exercise: relationship of cortisol to circulating thyroid hormones. Int J Endocrinol Metab. 2005; 1:18–24.26. Hesse V, Vilser C, Scheibe J, Jahreis G, Foley T. Thyroid hormone metabolism under extreme body exercises. Exp Clin Endocrinol. 1989; 94:82–88.
Article27. Hackney AC, Hodgdon JA, Hesslink R Jr, Trygg K. Thyroid hormone responses to military winter exercises in the Arctic region. Arctic Med Res. 1995; 54:82–90.28. Johannessen A, Hagen C, Galbo H. Prolactin, growth hormone, thyrotropin, 3,5,3'-triiodothyronine, and thyroxine responses to exercise after fat- and carbohydrate-enriched diet. J Clin Endocrinol Metab. 1981; 52:56–61.
Article29. Semple CG, Thomson JA, Beastall GH. Endocrine responses to marathon running. Br J Sports Med. 1985; 19:148–151.
Article30. Hackney AC, Dobridge JD. Thyroid hormones and the interrelationship of cortisol and prolactin: influence of prolonged, exhaustive exercise. Endokrynol Pol. 2009; 60:252–257.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Thyroid Stimulating Hormone on Bone Metabolism
- Clinical Characteristics and Treatments of Patients with TSH Secreting Pituitary Adenoma
- A Case of Acromegaly Caused by Mixed Gangliocytoma-Adenoma of the Pituitary Gland
- Hypopituitarism Presenting as Osteoporotic Fracture after Cured Tuberculous Meningitis
- A Case of Ioslated TSH Deficiency