Korean J Ophthalmol.
2018 Dec;32(6):459-469. 10.3341/kjo.2018.0004.
Peripapillary Retinal Nerve Fiber Layer Thicknesses Did Not Change in Long-term Hydroxychloroquine Users
- Affiliations
-
- 1Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sangjinkim@skku.edu
- 2Division of Rheumatology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2427960
- DOI: http://doi.org/10.3341/kjo.2018.0004
Abstract
- PURPOSE
To evaluate changes in the peripapillary retinal nerve fiber layer (RNFL) thicknesses using spectral-domain optical coherence tomography (SD-OCT) in hydroxychloroquine (HCQ) users.
METHODS
The medical records of HCQ users were retrospectively reviewed. In these HCQ users, an automated perimetry, fundus autofluorescence photography, and SD-OCT with peripapillary RNFL thickness measurements were performed. The peripapillary RNFL thicknesses were compared between the HCQ users and the control groups. The relationships between the RNFL thicknesses and the duration or cumulative dosage of HCQ use were analyzed.
RESULTS
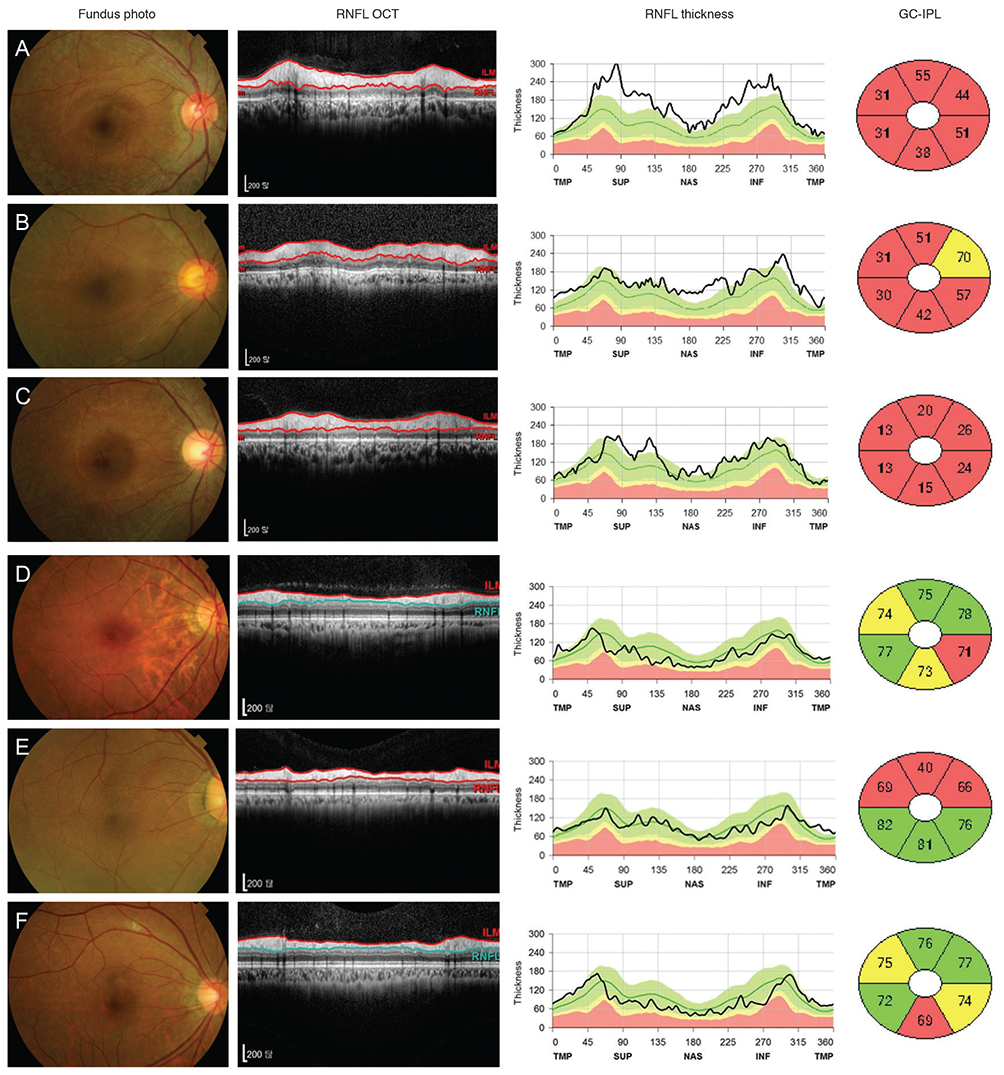
This study included 77 HCQ users and 20 normal controls. The mean duration of HCQ usage was 63.6 ± 38.4 months, and the cumulative dose of HCQ was 528.1 ± 3.44 g. Six patients developed HCQ retinopathy. Global and six sectoral RNFL thicknesses of the HCQ users did not significantly decrease compared to those of the normal controls. No significant correlation was found between the RNFL thickness and the duration of use or cumulative dose. The eyes of those with HCQ retinopathy had temporal peripapillary RNFL thicknesses significantly greater than that of normal controls.
CONCLUSIONS
The peripapillary RNFL thicknesses did not change in the HCQ users and did not correlate with the duration of HCQ use or cumulative doses of HCQ. RNFL thickness is not a useful biomarker for the early detection of HCQ retinal toxicity.
Keyword
MeSH Terms
Figure
Reference
-
1. Bernstein HN. Chloroquine ocular toxicity. Surv Ophthalmol. 1967; 12:415–447.2. Mavrikakis I, Sfikakis PP, Mavrikakis E, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003; 110:1321–1326.3. Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016; 123:1386–1394.
Article4. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014; 132:1453–1460.
Article5. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015; 122:110–116.
Article6. Marmor MF, Kellner U, Lai TY, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011; 118:415–422.
Article7. Marmor MF, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014; 132:1105–1112.
Article8. Hallberg A, Naeser P, Andersson A. Effects of long-term chloroquine exposure on the phospholipid metabolism in retina and pigment epithelium of the mouse. Acta Ophthalmol (Copenh). 1990; 68:125–130.
Article9. Rosenthal AR, Kolb H, Bergsma D, et al. Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci. 1978; 17:1158–1175.10. Bonanomi MT, Dantas NC, Medeiros FA. Retinal nerve fibre layer thickness measurements in patients using chloroquine. Clin Exp Ophthalmol. 2006; 34:130–136.
Article11. Xiaoyun MA, Dongyi HE, Linping HE. Assessing chloroquine toxicity in RA patients using retinal nerve fibre layer thickness, multifocal electroretinography and visual field test. Br J Ophthalmol. 2010; 94:1632–1636.
Article12. Pasadhika S, Fishman GA. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye (Lond). 2010; 24:340–346.
Article13. Tan BB, Natividad M, Chua KC, Yip LW. Comparison of retinal nerve fiber layer measurement between 2 spectral domain OCT instruments. J Glaucoma. 2012; 21:266–273.
Article14. Asrani S, Essaid L, Alder BD, Santiago-Turla C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol. 2014; 132:396–402.
Article15. Wheat JL, Rangaswamy NV, Harwerth RS. Correlating RNFL thickness by OCT with perimetric sensitivity in glaucoma patients. J Glaucoma. 2012; 21:95–101.
Article16. Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006; 59:963–969.
Article17. Ratchford JN, Quigg ME, Conger A, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009; 73:302–308.
Article18. Lee MG, Kim SJ, Ham DI, et al. Macular retinal ganglion cell-inner plexiform layer thickness in patients on hydroxychloroquine therapy. Invest Ophthalmol Vis Sci. 2014; 56:396–402.
Article19. Uslu H, Gurler B, Yildirim A, et al. Effect of hydroxychloroquine on the retinal layers: a quantitative evaluation with spectral-domain optical coherence tomography. J Ophthalmol. 2016; 2016:8643174.
Article20. Hoesl LM, Tornow RP, Schrems WA, et al. Glaucoma diagnostic performance of GDxVCC and spectralis OCT on eyes with atypical retardation pattern. J Glaucoma. 2013; 22:317–324.
Article21. Lemij HG. The value of polarimetry in the evaluation of the optic nerve in glaucoma. Curr Opin Ophthalmol. 2001; 12:138–142.
Article22. Schallenberg M, Dekowski D, Kremmer S, et al. Comparison of Spectralis-OCT, GDxVCC and GDxECC in assessing retinal nerve fiber layer (RNFL) in glaucomatous patients. Graefes Arch Clin Exp Ophthalmol. 2013; 251:1343–1353.
Article23. Bagga H, Greenfield DS, Feuer W, Knighton RW. Scanning laser polarimetry with variable corneal compensation and optical coherence tomography in normal and glaucomatous eyes. Am J Ophthalmol. 2003; 135:521–529.
Article24. Sehi M, Ume S, Greenfield DS. Scanning laser polarimetry with enhanced corneal compensation and optical coherence tomography in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2007; 48:2099–2104.
Article25. Kook MS, Cho HS, Seong M, Choi J. Scanning laser polarimetry using variable corneal compensation in the detection of glaucoma with localized visual field defects. Ophthalmology. 2005; 112:1970–1978.
Article26. Garas A, Toth M, Vargha P, Hollo G. Comparison of repeatability of retinal nerve fiber layer thickness measurement made using the RTVue Fourier-domain optical coherence tomograph and the GDx scanning laser polarimeter with variable or enhanced corneal compensation. J Glaucoma. 2010; 19:412–417.
Article27. Oh JH, Kim YY. Scanning laser polarimetry and optical coherence tomography for detection of retinal nerve fiber layer defects. Korean J Ophthalmol. 2009; 23:169–175.
Article28. Bagga H, Greenfield DS, Knighton RW. Scanning laser polarimetry with variable corneal compensation: identification and correction for corneal birefringence in eyes with macular disease. Invest Ophthalmol Vis Sci. 2003; 44:1969–1976.
Article29. Giani A, Cigada M, Esmaili DD, et al. Artifacts in automatic retinal segmentation using different optical coherence tomography instruments. Retina. 2010; 30:607–616.
Article30. Sull AC, Vuong LN, Price LL, et al. Comparison of spectral/Fourier domain optical coherence tomography instruments for assessment of normal macular thickness. Retina. 2010; 30:235–245.
Article31. Anastasakis A, Genead MA, McAnany JJ, Fishman GA. Evaluation of retinal nerve fiber layer thickness in patients with retinitis pigmentosa using spectral-domain optical coherence tomography. Retina. 2012; 32:358–363.
Article32. Hwang YH, Kim SW, Kim YY, et al. Optic nerve head, retinal nerve fiber layer, and macular thickness measurements in young patients with retinitis pigmentosa. Curr Eye Res. 2012; 37:914–920.
Article33. Walia S, Fishman GA. Retinal nerve fiber layer analysis in RP patients using Fourier-domain OCT. Invest Ophthalmol Vis Sci. 2008; 49:3525–3528.
Article34. Han J, Lee K, Rhiu S, et al. Linezolid-associated optic neuropathy in a patient with drug-resistant tuberculosis. J Neuroophthalmol. 2013; 33:316–318.
Article35. Barboni P, Carbonelli M, Savini G, et al. Natural history of Leber's hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010; 117:623–627.
Article36. Hood DC, Lin CE, Lazow MA, et al. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009; 50:2328–2336.
Article37. Jacobson SG, Sumaroka A, Aleman TS, et al. Evidence for retinal remodelling in retinitis pigmentosa caused by PDE6B mutation. Br J Ophthalmol. 2007; 91:699–701.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Retinal Herniation through Peripapillary Pit Resulting in Retinal Nerve Fiber Layer Defect
- Associations of Peripapillary Retinal Nerve Fiber Layer and Macular Retinal Layer Thickness with Serum Homocysteine Concentration
- Thicknesses of Macular Retinal Layer and Peripapillary Retinal Nerve Fiber Layer in Patients with Hyperopic Anisometropic Amblyopia
- Reproducibility of Retinal Nerve Fiber Layer Thickness Evaluation by Nerve Fiber Analyzer
- Thicknesses of the Fovea and Retinal Nerve Fiber Layer in Amblyopic and Normal Eyes in Children