Korean J Radiol.
2017 Apr;18(2):383-391. 10.3348/kjr.2017.18.2.383.
Assessment of Arterial Wall Enhancement for Differentiation of Parent Artery Disease from Small Artery Disease: Comparison between Histogram Analysis and Visual Analysis on 3-Dimensional Contrast-Enhanced T1-Weighted Turbo Spin Echo MR Images at 3T
- Affiliations
-
- 1Department of Radiology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. hschoi@catholic.ac.kr
- 2Department of Neurology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 3Department of Neurosurgery, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2427951
- DOI: http://doi.org/10.3348/kjr.2017.18.2.383
Abstract
OBJECTIVE
The purpose of this study was to compare the histogram analysis and visual scores in 3T MRI assessment of middle cerebral arterial wall enhancement in patients with acute stroke, for the differentiation of parent artery disease (PAD) from small artery disease (SAD).
MATERIALS AND METHODS
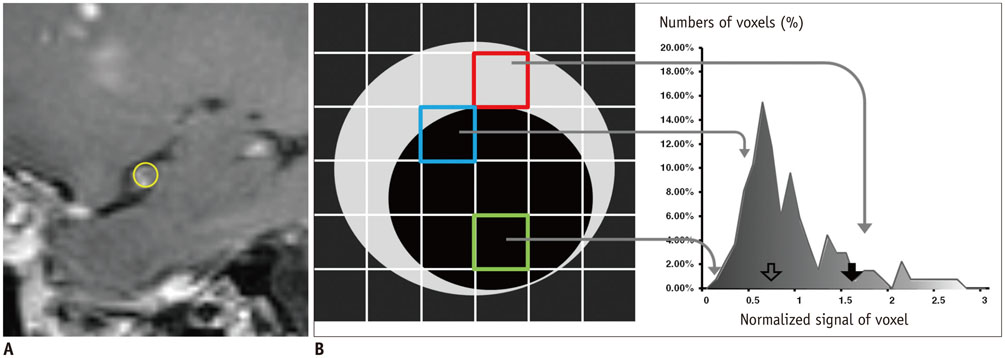
Among the 82 consecutive patients in a tertiary hospital for one year, 25 patients with acute infarcts in middle cerebral artery (MCA) territory were included in this study including 15 patients with PAD and 10 patients with SAD. Three-dimensional contrast-enhanced T1-weighted turbo spin echo MR images with black-blood preparation at 3T were analyzed both qualitatively and quantitatively. The degree of MCA stenosis, and visual and histogram assessments on MCA wall enhancement were evaluated. A statistical analysis was performed to compare diagnostic accuracy between qualitative and quantitative metrics.
RESULTS
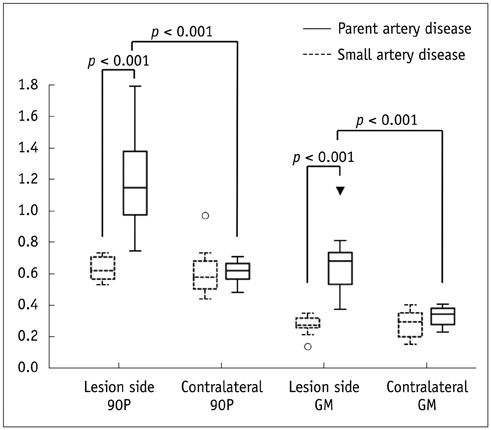
The degree of stenosis, visual enhancement score, geometric mean (GM), and the 90th percentile (90P) value from the histogram analysis were significantly higher in PAD than in SAD (p = 0.006 for stenosis, < 0.001 for others). The receiver operating characteristic curve area of GM and 90P were 1 (95% confidence interval [CI], 0.86-1.00).
CONCLUSION
A histogram analysis of a relevant arterial wall enhancement allows differentiation between PAD and SAD in patients with acute stroke within the MCA territory.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
Area Under Curve
Constriction, Pathologic/pathology
Female
Humans
Image Enhancement
Imaging, Three-Dimensional
Magnetic Resonance Imaging/*methods
Male
Middle Aged
Middle Cerebral Artery/diagnostic imaging/physiology
Myocardial Infarction/*diagnosis
ROC Curve
Retrospective Studies
Tertiary Care Centers
Figure
Cited by 1 articles
-
Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.
Reference
-
1. Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990; 40:1541–1545.2. Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996; 27:1974–1980.3. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.4. Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol. 2013; 34:299–304.5. Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. 2013; 44:287–292.6. Wardlaw JM. What is a lacune? Stroke. 2008; 39:2921–2922.7. Kim JS, Yoon Y. Single subcortical infarction associated with parental arterial disease: important yet neglected sub-type of atherothrombotic stroke. Int J Stroke. 2013; 8:197–203.8. Yoon Y, Lee DH, Kang DW, Kwon SU, Kim JS. Single subcortical infarction and atherosclerotic plaques in the middle cerebral artery: high-resolution magnetic resonance imaging findings. Stroke. 2013; 44:2462–2467.9. Chimowitz M, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011; 365:993–1003.10. Turan TN, Cotsonis G, Lynn MJ, Wooley RH, Swanson S, Williams JE, et al. Intracranial stenosis: impact of randomized trials on treatment preferences of US neurologists and neurointerventionists. Cerebrovasc Dis. 2014; 37:203–211.11. Ding D, Starke RM, Crowley RW, Liu KC. Role of stenting for intracranial atherosclerosis in the post-SAMMPRIS era. Biomed Res Int. 2013; 2013:304320.12. Klein IF, Lavallée PC, Touboul PJ, Schouman-Claeys E, Amarenco P. In vivo middle cerebral artery plaque imaging by high-resolution MRI. Neurology. 2006; 67:327–329.13. Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-Tesla contrast-enhanced MRI. Neurology. 2009; 72:627–634.14. Klein IF, Lavallée PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke. 2010; 41:1405–1409.15. Qiao Y, Zeiler SR, Mirbagheri S, Leigh R, Urrutia V, Wityk R, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014; 271:534–542.16. Heye T, Merkle EM, Reiner CS, Davenport MS, Horvath JJ, Feuerlein S, et al. Reproducibility of dynamic contrast-enhanced MR imaging Part II Comparison of intra- and interobserver variability with manual region of interest placement versus semiautomatic lesion segmentation and histogram analysis. Radiology. 2013; 266:812–821.17. Lee DK, Kim JS, Kwon SU, Yoo SH, Kang DW. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: early diffusion-weighted imaging study. Stroke. 2005; 36:2583–2588.18. Nguyen TD, De Rochefort L, Spincemaille P, Cham MD, Weinsaft JW, Prince MR, et al. Effective motion-sensitizing magnetization preparation for black blood magnetic resonance imaging of the heart. J Magn Reson Imaging. 2008; 28:1092–1100.19. van der Kolk AG, Zwanenburg JJ, Brundel M, Biessels GJ, Visser F, Luijten PR, et al. Intracranial vessel wall imaging at 7.0-T MRI. Stroke. 2011; 42:2478–2484.20. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000; 21:643–646.21. Natori T, Sasaki M, Miyoshi M, Ohba H, Katsura N, Yamaguchi M, et al. Evaluating middle cerebral artery atherosclerotic lesions in acute ischemic stroke using magnetic resonance T1-weighted 3-dimensional vessel wall imaging. J Stroke Cerebrovasc Dis. 2014; 23:706–711.22. Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996; 312:1079.23. Dieleman N, van der Kolk AG, Zwanenburg JJ, Harteveld AA, Biessels GJ, Luijten PR, et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions. Circulation. 2014; 130:192–201.24. Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008; 39:1142–1147.25. Ryu CW, Jahng GH, Kim EJ, Choi WS, Yang DM. High resolution wall and lumen MRI of the middle cerebral arteries at 3 Tesla. Cerebrovasc Dis. 2009; 27:433–442.26. Dieleman N, Yang W, Abrigo JM, Chu WC, van der Kolk AG, Siero JC, et al. Magnetic resonance imaging of plaque morphology, burden, and distribution in patients with symptomatic middle cerebral artery stenosis. Stroke. 2016; 47:1797–1802.27. Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging. 2011; 34:22–30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MR of Normal Pancreas: Comparison of Five Pulse Sequences and Enhancing Patterns on Dynamic Imaging
- Contrast-Enhanced Turbo Spin-Echo(TSE) T1-weighted Imaging: Improved Contrast of Enhancing Lesions
- Characterization of Focal Liver Lesions with Superparamagnetic Iron Oxide-Enhanced MR Imaging: Value of Distributional Phase T1-Weighted Imaging
- Small Focal Hepatic Lesions <=1 cm: Their Detection and Characterization with Performing Diffusion-Weighted Sensitivity-Encoding versus SPIO-Enhanced 3T MR Imaging
- Meniscal Tears of the Knee: Diagnosis with Fast Spin-Echo MR Imaging and Role of Gadolinium-Enhancement