Korean J Radiol.
2017 Oct;18(5):844-851. 10.3348/kjr.2017.18.5.844.
Optimal Factors of Diffusion Tensor Imaging Predicting Corticospinal Tract Injury in Patients with Brain Tumors
- Affiliations
-
- 1Department of Radiology, Yixing Hospital Affiliated of Jiangsu University, Yixing 214200, China.
- 2Department of Radiology, First Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710061, China. xjtuzhangming@gmail.com
- 3Department of Medical Imaging, School of Medicine, Jiangsu University, Zhenjiang 212013, China.
- KMID: 2427221
- DOI: http://doi.org/10.3348/kjr.2017.18.5.844
Abstract
OBJECTIVE
To identify the optimal factors in diffusion tensor imaging for predicting corticospinal tract (CST) injury caused by brain tumors.
MATERIALS AND METHODS
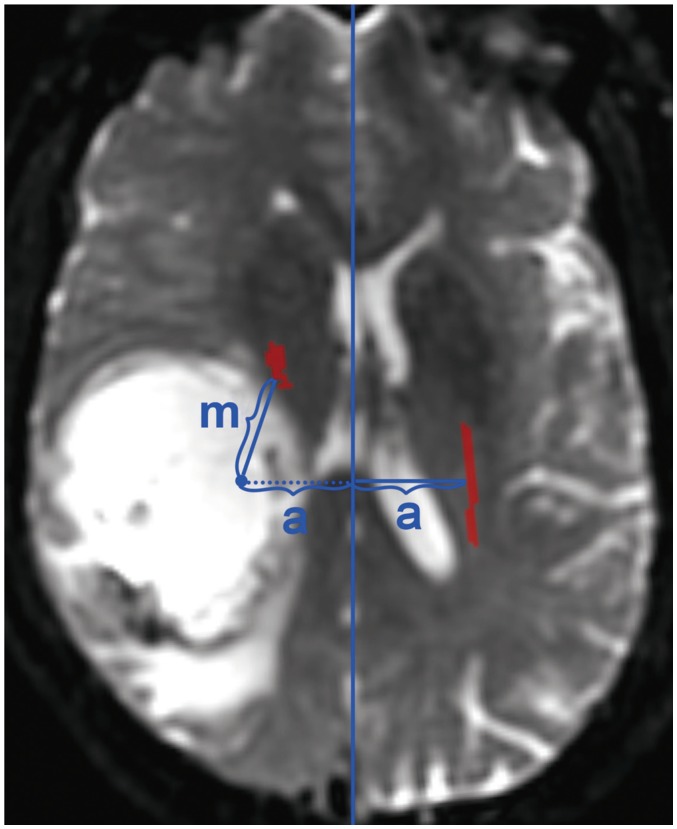
This prospective study included 33 patients with motor weakness and 64 patients with normal motor function. The movement of the CST, minimum distance between the CST and the tumor, and relative fractional anisotropy (rFA) of the CST on diffusion tensor imaging, were compared between patients with motor weakness and normal function. Logistic regression analysis was used to obtain the optimal factor predicting motor weakness.
RESULTS
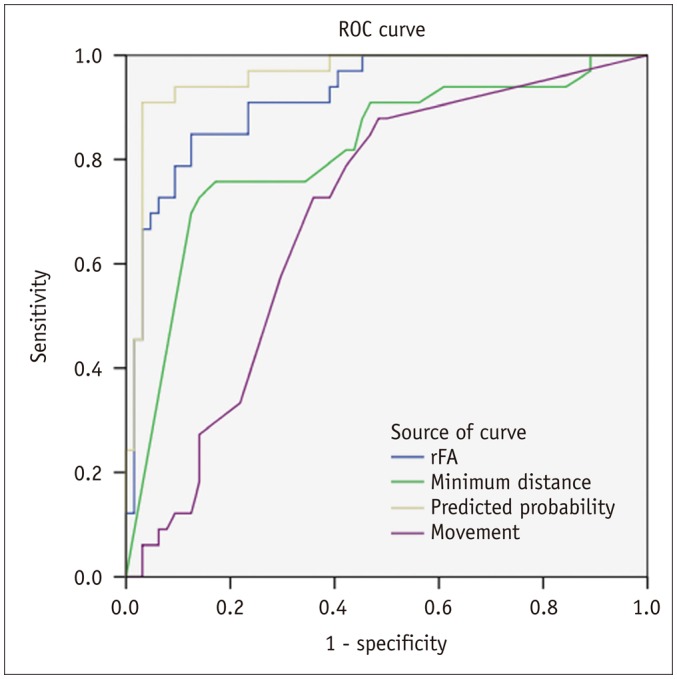
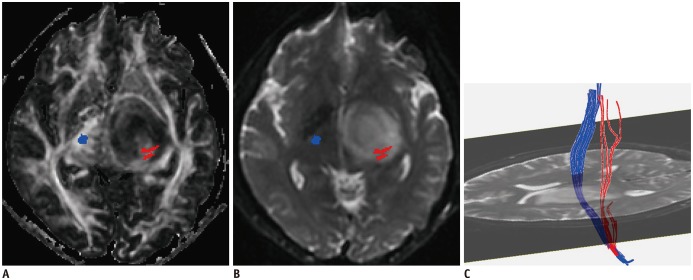
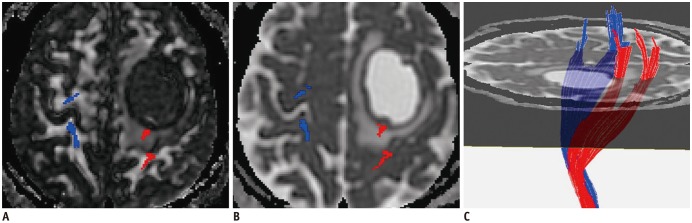
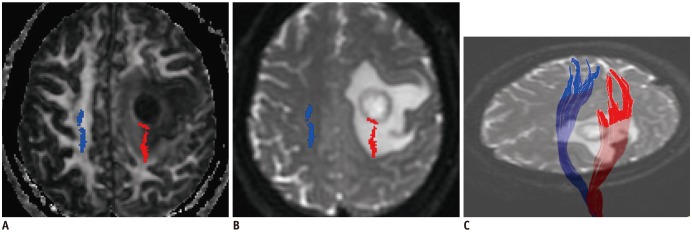
In patients with motor weakness, the displacement (8.44 ± 6.64 mm) of the CST (p = 0.009), minimum distance (3.98 ± 7.49 mm) between the CST and tumor (p < 0.001), and rFA (0.83 ± 0.11) of the CST (p < 0.001) were significantly different from those of the normal group (4.64 ± 6.65 mm, 14.87 ± 12.04 mm, and 0.98 ± 0.05, respectively) (p = 0.009, p < 0.001, and p < 0.001). The frequencies of patients with the CST passing through the tumor (6%, p = 0.002), CST close to the tumor (23%, p < 0.001), CST close to a malignant tumor (high grade glioma, metastasis, or lymphoma) (19%, p < 0.001), and CST passing through infiltrating edema (19%, p < 0.001) in the motor weakness group, were significantly different from those of the patients with normal motor function (0, 8, 1, and 10%, respectively). Logistic regression analysis showed that decreased rFA and CST close to a malignant tumor were effective variables related to motor weakness.
CONCLUSION
Decreased fractional anisotropy, combined with closeness of a malignant tumor to the CST, is the optimal factor in predicting CST injury caused by a brain tumor.
MeSH Terms
Figure
Cited by 2 articles
-
Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.Clinical Uses of Diffusion Tensor Imaging Fiber Tracking Merged Neuronavigation with Lesions Adjacent to Corticospinal Tract : A Retrospective Cohort Study
Qi Yu, Kun Lin, Yunhui Liu, Xinxing Li
J Korean Neurosurg Soc. 2020;63(2):248-260. doi: 10.3340/jkns.2019.0046.
Reference
-
1. Dimou S, Battisti RA, Hermens DF, Lagopoulos J. A systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection. Neurosurg Rev. 2013; 36:205–214. discussion 214. PMID: 23187966.
Article2. Yu CS, Li KC, Xuan Y, Ji XM, Qin W. Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and postoperative assessment. Eur J Radiol. 2005; 56:197–204. PMID: 15916876.
Article3. Itagiba VGA, Borges R, da Cruz LCH Jr, Furtado AD, Domingues RC, Gasparetto EL. Use of diffusion tensor magnetic resonance imaging in the assessment of patterns of white matter involvement in patients with brain tumors: is it useful in the differential diagnosis? Radiol Bras. 2010; 43:362–368.4. Laundre BJ, Jellison BJ, Badie B, Alexander AL, Field AS. Diffusion tensor imaging of the corticospinal tract before and after mass resection as correlated with clinical motor findings: preliminary data. AJNR Am J Neuroradiol. 2005; 26:791–796. PMID: 15814922.5. Kovanlikaya I, Firat Z, Kovanlikaya A, Uluğ AM, Cihangiroglu MM, John M, et al. Assessment of the corticospinal tract alterations before and after resection of brainstem lesions using diffusion tensor imaging (DTI) and tractography at 3T. Eur J Radiol. 2011; 77:383–391. PMID: 19767164.
Article6. Min ZG, Rana N, Niu C, Ji HM, Zhang M. Does diffusion tensor tractography of the corticospinal tract correctly reflect motor function? Med Princ Pract. 2014; 23:174–176. PMID: 23949557.
Article7. Kwon YH, Son SM, Lee J, Bai DS, Jang SH. Combined study of transcranial magnetic stimulation and diffusion tensor tractography for prediction of motor outcome in patients with corona radiata infarct. J Rehabil Med. 2011; 43:430–434. PMID: 21403983.
Article8. Kim CH, Chung CK, Kim JS, Jahng TA, Lee JH, Song IC. Use of diffusion tensor imaging to evaluate weakness. J Neurosurg. 2007; 106:111–118.
Article9. Morita N, Wang S, Kadakia P, Chawla S, Poptani H, Melhem ER. Diffusion tensor imaging of the corticospinal tract in patients with brain neoplasms. Magn Reson Med Sci. 2011; 10:239–243. PMID: 22214908.
Article10. Stadlbauer A, Nimsky C, Gruber S, Moser E, Hammen T, Engelhorn T, et al. Changes in fiber integrity, diffusivity, and metabolism of the pyramidal tract adjacent to gliomas: a quantitative diffusion tensor fiber tracking and MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2007; 28:462–469. PMID: 17353313.11. Puig J, Pedraza S, Blasco G, Daunis-I-Estadella J, Prats A, Prados F, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol. 2010; 31:1324–1330. PMID: 20299434.
Article12. De Belder FE, Oot AR, Van Hecke W, Venstermans C, Menovsky T, Van Marck V, et al. Diffusion tensor imaging provides an insight into the microstructure of meningiomas, high-grade gliomas, and peritumoral edema. J Comput Assist Tomogr. 2012; 36:577–582. PMID: 22992609.
Article13. Kim MS, Chung CK, Jung HW, Park CK, Kim CH, Kim JS. Preoperative weakness and demyelination of the corticospinal tract in meningioma patients: changes in diffusion parameters using diffusion tensor imaging. J Korean Neurosurg Soc. 2014; 55:267–272. PMID: 25132933.14. Cuthbert SC, Goodheart GJ Jr. On the reliability and validity of manual muscle testing: a literature review. Chiropr Osteopat. 2007; 15:4. PMID: 17341308.
Article15. Kim M, Kim HS. Emerging techniques in brain tumor imaging: what radiologists need to know. Korean J Radiol. 2016; 17:598–619. PMID: 27587949.
Article16. Berman JI, Berger MS, Mukherjee P, Henry RG. Diffusiontensor imaging-guided tracking of fibers of the pyramidal tract combined with intraoperative cortical stimulation mapping in patients with gliomas. J Neurosurg. 2004; 101:66–72. PMID: 15255253.
Article17. Hendler T, Pianka P, Sigal M, Kafri M, Ben-Bashat D, Constantini S, et al. Delineating gray and white matter involvement in brain lesions: three-dimensional alignment of functional magnetic resonance and diffusion-tensor imaging. J Neurosurg. 2003; 99:1018–1027. PMID: 14705730.18. Mori S, Frederiksen K, van Zijl PC, Stieltjes B, Kraut MA, Solaiyappan M, et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol. 2002; 51:377–380. PMID: 11891834.
Article19. Clark CA, Barrick TR, Murphy MM, Bell BA. White matter fiber tracking in patients with space-occupying lesions of the brain: a new technique for neurosurgical planning? Neuroimage. 2003; 20:1601–1608. PMID: 14642471.
Article20. Yamada K, Kizu O, Mori S, Ito H, Nakamura H, Yuen S, et al. Brain fiber tracking with clinically feasible diffusion-tensor MR imaging: initial experience. Radiology. 2003; 227:295–301. PMID: 12668749.
Article21. Bagadia A, Purandare H, Misra BK, Gupta S. Application of magnetic resonance tractography in the perioperative planning of patients with eloquent region intra-axial brain lesions. J Clin Neurosci. 2011; 18:633–639. PMID: 21371893.
Article22. Mathias J, Koessler L, Brissart H, Foscolo S, Schmitt E, Bracard S, et al. Giant cystic widening of Virchow-Robin spaces: an anatomofunctional study. AJNR Am J Neuroradiol. 2007; 28:1523–1525. PMID: 17846204.
Article23. Young RJ, Lee V, Peck KK, Sierra T, Zhang Z, Jacks LM, et al. Diffusion tensor imaging and tractography of the corticospinal tract in the presence of enlarged Virchow-Robin spaces. J Neuroimaging. 2014; 24:79–82. PMID: 22082139.
Article24. Ng WH, Cheong DL, Khu KJ, Venkatesh G, Ng YK, Lim CC. Diffusion tensor tractography: corticospinal tract fiber reduction is associated with temporary hemiparesis in benign extracerebral lesions. Neurosurgery. 2008; 63:452–458. discussion 458-459. PMID: 18812956.25. Yamahara T, Numa Y, Oishi T, Kawaguchi T, Seno T, Asai A, et al. Morphological and flow cytometric analysis of cell infiltration in glioblastoma: a comparison of autopsy brain and neuroimaging. Brain Tumor Pathol. 2010; 27:81–87. PMID: 21046309.
Article26. Goebell E, Paustenbach S, Vaeterlein O, Ding XQ, Heese O, Fiehler J, et al. Low-grade and anaplastic gliomas: differences in architecture evaluated with diffusion-tensor MR imaging. Radiology. 2006; 239:217–222. PMID: 16484348.
Article27. Schlüter M, Stieltjes B, Hahn HK, Rexilius J, Konrad-verse O, Peitgen HO. Detection of tumour infiltration in axonal fibre bundles using diffusion tensor imaging. Int J Med Robot. 2005; 1:80–86.
Article28. Reich DS, Smith SA, Jones CK, Zackowski KM, van Zijl PC, Calabresi PA, et al. Quantitative characterization of the corticospinal tract at 3T. AJNR Am J Neuroradiol. 2006; 27:2168–2178. PMID: 17110689.29. Yamada K, Kizu O, Kubota T, Ito H, Matsushima S, Oouchi H, et al. The pyramidal tract has a predictable course through the centrum semiovale: a diffusion-tensor based tractography study. J Magn Reson Imaging. 2007; 26:519–524. PMID: 17729353.
Article30. Stadlbauer A, Nimsky C, Buslei R, Salomonowitz E, Hammen T, Buchfelder M, et al. Diffusion tensor imaging and optimized fiber tracking in glioma patients: histopathologic evaluation of tumor-invaded white matter structures. Neuroimage. 2007; 34:949–956. PMID: 17166744.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Challenge of Diffusion Tensor Imaging in a Patient With Hemiplegia After Traumatic Brain Injury
- Central Pain Due to Traumatic Axonal Injury of the Spinothalamic Tract in Patients with Mild Traumatic Brain Injury
- Analysis of Corticospinal Tract Injury by Using the Diffusion Tensor Imaging of 3.0 T Magnetic Resonance in Patients with Hypertensive Intracerebral Hemorrhage
- Gait Characteristic in a Stroke Patient with an Intact Corticospinal Tract and Corticoreticular Pathway: A Case Study
- Characteristics of Corticospinal Tract Area According to Pontine Level