Korean J Radiol.
2017 Oct;18(5):835-843. 10.3348/kjr.2017.18.5.835.
Correlation between Intravoxel Incoherent Motion Magnetic Resonance Imaging Derived Metrics and Serum Soluble CD40 Ligand Level in an Embolic Canine Stroke Model
- Affiliations
-
- 1Department of Radiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China. liusheng@njmu.edu.cn
- KMID: 2427220
- DOI: http://doi.org/10.3348/kjr.2017.18.5.835
Abstract
OBJECTIVE
To determine the relationship between intravoxel incoherent motion (IVIM) imaging derived quantitative metrics and serum soluble CD40 ligand (sCD40L) level in an embolic canine stroke model.
MATERIALS AND METHODS
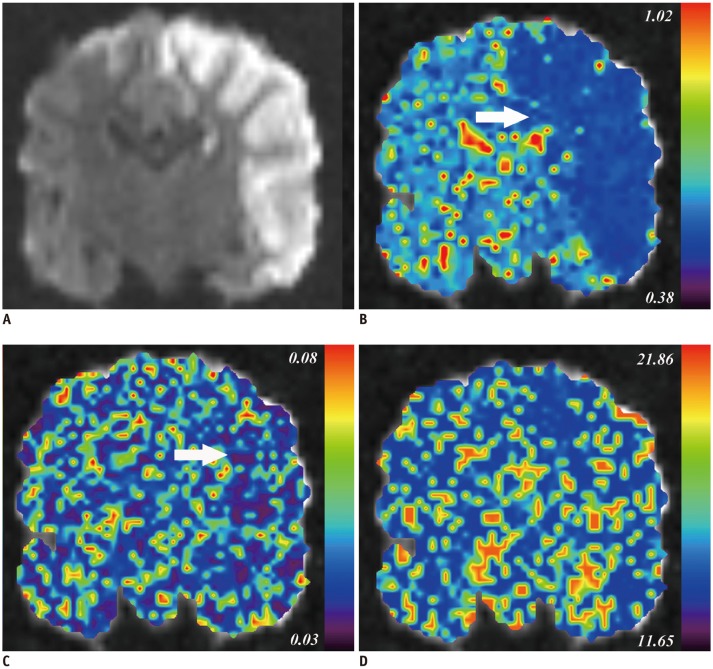
A middle cerebral artery occlusion model was established in 24 beagle dogs. Experimental dogs were divided into low- and high-sCD40L group according to serum sCD40L level at 4.5 hours after establishing the model. IVIM imaging was scanned at 4.5 hours after model establishment using 10 b values ranging from 0 to 900 s/mm². Quantitative metrics diffusion coefficient (D), pseudodiffusion coefficient (D*), and perfusion fraction (f) of ischemic lesions were calculated. Quantitative metrics of ischemic lesions were normalized by contralateral hemisphere using the following formula: normalized D = D(stroke) / D(contralateral). Differences in IVIM metrics between the low- and high-sCD40L groups were compared using t test. Pearson's correlation analyses were performed to determine the relationship between IVIM metrics and serum sCD40L level.
RESULTS
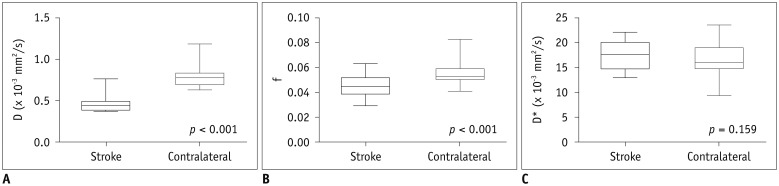
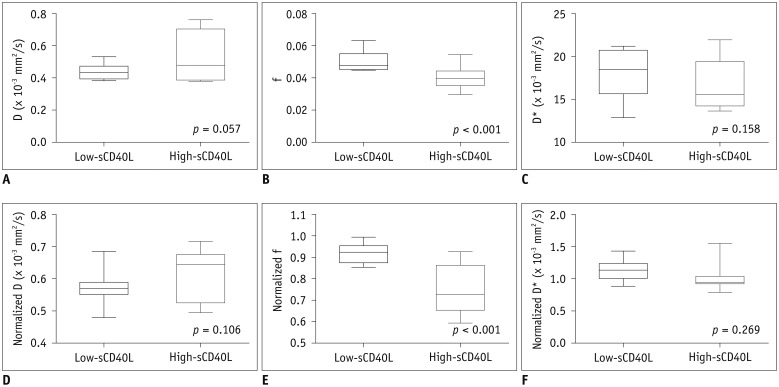
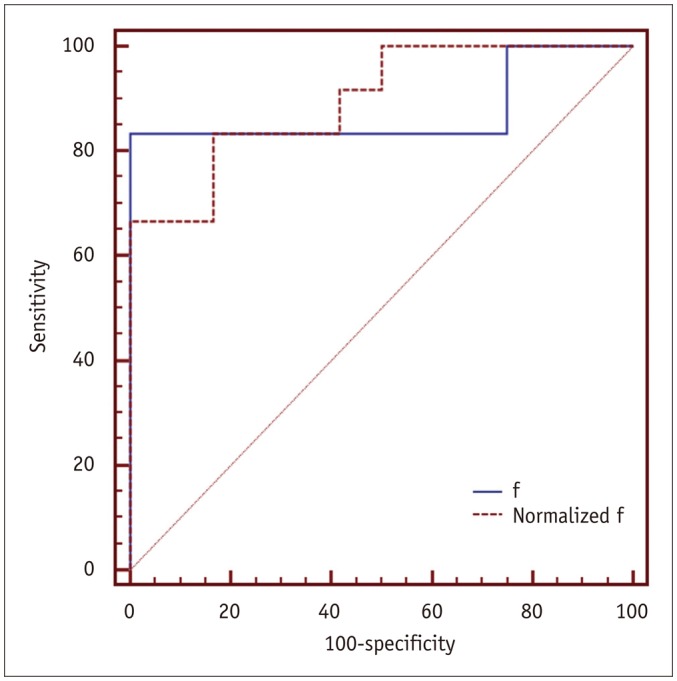
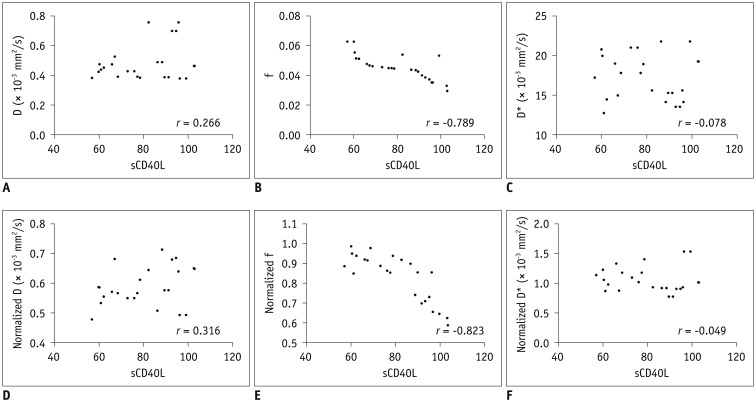
The high-sCD40L group showed significantly lower f and normalized f values than the low-sCD40L group (f, p < 0.001; normalized f, p < 0.001). There was no significant difference in D*, normalized D*, D, or normalized D value between the two groups (All p > 0.05). Both f and normalized f values were negatively correlated with serum sCD40L level (f, r = −0.789, p < 0.001; normalized f, r = −0.823, p < 0.001). However, serum sCD40L level had no significant correlation with D*, normalized D*, D, or normalized D (All p > 0.05).
CONCLUSION
The f value derived from IVIM imaging was negatively correlated with serum sCD40L level. f value might serve as a potential imaging biomarker to assess the formation of microvascular thrombosis in hyperacute period of ischemic stroke.
Keyword
MeSH Terms
Figure
Reference
-
1. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030. PMID: 25671798.2. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 359:1317–1329. PMID: 18815396.
Article3. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015; 372:1009–1018. PMID: 25671797.
Article4. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 372:2285–2295. PMID: 25882376.
Article5. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; 372:2296–2306. PMID: 25882510.
Article6. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20. PMID: 25517348.7. Soares BP, Tong E, Hom J, Cheng SC, Bredno J, Boussel L, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke. 2010; 41:e34–e40. PMID: 19910542.
Article8. Desilles JP, Loyau S, Syvannarath V, Gonzalez-Valcarcel J, Cantier M, Louedec L, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. 2015; 46:3241–3248. PMID: 26443832.
Article9. Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005; 111:1690–1696. PMID: 15795333.
Article10. Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab. 2004; 24:907–915. PMID: 15362721.
Article11. Federau C, Maeder P, O'Brien K, Browaeys P, Meuli R, Hagmann P. Quantitative measurement of brain perfusion with intravoxel incoherent motion MR imaging. Radiology. 2012; 265:874–881. PMID: 23074258.
Article12. Federau C, Sumer S, Becce F, Maeder P, O'Brien K, Meuli R, et al. Intravoxel incoherent motion perfusion imaging in acute stroke: initial clinical experience. Neuroradiology. 2014; 56:629–635. PMID: 24838807.
Article13. Federau C, O'Brien K, Meuli R, Hagmann P, Maeder P. Measuring brain perfusion with intravoxel incoherent motion (IVIM): initial clinical experience. J Magn Reson Imaging. 2014; 39:624–632. PMID: 24068649.
Article14. Hu LB, Hong N, Zhu WZ. Quantitative measurement of cerebral perfusion with intravoxel incoherent motion in acute ischemia stroke: initial clinical experience. Chin Med J (Engl). 2015; 128:2565–2569. PMID: 26415791.15. Yao Y, Zhang S, Tang X, Zhang S, Shi J, Zhu W, et al. Intravoxel incoherent motion diffusion-weighted imaging in stroke patients: initial clinical experience. Clin Radiol. 2016; 71:938.e11–938.e16.
Article16. Suo S, Cao M, Zhu W, Li L, Li J, Shen F, et al. Stroke assessment with intravoxel incoherent motion diffusion-weighted MRI. NMR Biomed. 2016; 29:320–328. PMID: 26748572.
Article17. Liu S, Hu WX, Zu QQ, Lu SS, Xu XQ, Sun L, et al. A novel embolic stroke model resembling lacunar infarction following proximal middle cerebral artery occlusion in beagle dogs. J Neurosci Methods. 2012; 209:90–96. PMID: 22722089.
Article18. Xu XQ, Zu QQ, Lu SS, Cheng QG, Yu J, Sheng Y, et al. Use of FLAIR imaging to identify onset time of cerebral ischemia in a canine model. AJNR Am J Neuroradiol. 2014; 35:311–316. PMID: 23928141.
Article19. Xu XQ, Su GY, Liu J, Hu H, Hong XN, Shi HB, et al. Intravoxel incoherent motion MR imaging measurements of the bilateral parotid glands at 3.0-T MR: effect of age, gender and laterality in healthy adults. Br J Radiol. 2015; 88:20150646. PMID: 26449128.
Article20. Kim DY, Kim HS, Goh MJ, Choi CG, Kim SJ. Utility of intravoxel incoherent motion MR imaging for distinguishing recurrent metastatic tumor from treatment effect following gamma knife radiosurgery: initial experience. AJNR Am J Neuroradiol. 2014; 35:2082–2090. PMID: 24970548.
Article21. Xu XQ, Choi YJ, Sung YS, Yoon RG, Jang SW, Park JE, et al. Intravoxel incoherent motion MR imaging in the head and neck: correlation with dynamic contrast-enhanced MR imaging and diffusion-weighted imaging. Korean J Radiol. 2016; 17:641–649. PMID: 27587952.
Article22. Bisdas S, Braun C, Skardelly M, Schittenhelm J, Teo TH, Thng CH, et al. Correlative assessment of tumor microcirculation using contrast-enhanced perfusion MRI and intravoxel incoherent motion diffusion-weighted MRI: is there a link between them? NMR Biomed. 2014; 27:1184–1191. PMID: 25088433.
Article23. Suh CH, Kim HS, Lee SS, Kim N, Yoon HM, Choi CG, et al. Atypical imaging features of primary central nervous system lymphoma that mimics glioblastoma: utility of intravoxel incoherent motion MR imaging. Radiology. 2014; 272:504–513. PMID: 24697149.
Article24. Iima M, Reynaud O, Tsurugizawa T, Ciobanu L, Li JR, Geffroy F, et al. Characterization of glioma microcirculation and tissue features using intravoxel incoherent motion magnetic resonance imaging in a rat brain model. Invest Radiol. 2014; 49:485–490. PMID: 24619211.
Article25. Andreou A, Koh DM, Collins DJ, Blackledge M, Wallace T, Leach MO, et al. Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. Eur Radiol. 2013; 23:428–434. PMID: 23052642.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravoxel Incoherent Motion MR Imaging in the Head and Neck: Correlation with Dynamic Contrast-Enhanced MR Imaging and Diffusion-Weighted Imaging
- Comparative Study between ZOOMit and Conventional Intravoxel Incoherent Motion MRI for Assessing Parotid Gland Abnormalities in Patients with Early- or Mid-Stage Sjögren’s Syndrome
- Intravoxel Incoherent Motion Magnetic Resonance Imaging for Assessing Parotid Gland Tumors: Correlation and Comparison with Arterial Spin Labeling Imaging
- Correlation of the Speed of Enhancement of Hepatic Hemangiomas with Intravoxel Incoherent Motion MR Imaging
- RE: Distinguishing between Renal Cell Carcinoma and Fat Poor Angiomyolipoma in Diffusion-Weighted Imaging