Korean J Radiol.
2017 Dec;18(6):906-914. 10.3348/kjr.2017.18.6.906.
CT Enterography for Surveillance of Anastomotic Recurrence within 12 Months of Bowel Resection in Patients with Crohn's Disease: An Observational Study Using an 8-Year Registry
- Affiliations
-
- 1Department of Radiology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan 15355, Korea.
- 2Department of Gastroenterology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea.
- 3Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea. parksh.radiology@gmail.com
- 4Department of Colorectal Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea.
- KMID: 2427200
- DOI: http://doi.org/10.3348/kjr.2017.18.6.906
Abstract
OBJECTIVE
To investigate the diagnostic yield and accuracy of CT enterography (CTE) for early (< 12 postoperative months) surveillance of anastomotic recurrence after bowel resection for Crohn's disease (CD).
MATERIALS AND METHODS
We analyzed 88 adults (60 males and 28 females; mean age, 31.4 ± 9.6 years) who underwent bowel surgery for CD that created ileocolic anastomosis without enteric stoma, and underwent CTE for surveillance of CD recurrence/aggravation within 12 post-operative months. The CD activity index (CDAI) at the time of CTE was < 150 (i.e., clinically silent) in 51 patients, and ≥ 150 in 37 patients. Diagnostic yields of CTE regarding CD recurrence in the ileocolic anastomosis and extraluminal penetrating complications were determined. CTE-related step-up therapy was recorded. These outcomes were compared between the two CDAI groups after accounting for major risk factors for CD recurrence. In a subgroup of 31 patients who underwent both CTE and ileocolonoscopy within 1 month, CTE accuracy for anastomotic recurrence was assessed using the Rutgeerts scoring as the reference standard.
RESULTS
CTE diagnostic yield was 35.2% (31/88) for the anastomotic recurrence and 9.1% (8/88) for penetrating complications. 20.5% (18/88) of the patients underwent step-up therapy after CTE detection of anastomotic recurrence. These outcomes were not significantly different between CDAI < 150 and CDAI ≥ 150, except that CTE yield for extraluminal penetrating complications was significantly higher in CDAI ≥ 150 (16.2% [6/37] vs. 3.9% [2/51]; multivariable-adjusted p = 0.029). CTE showed 92.3% (12/13) sensitivity and 83.3% (15/18) specificity for anastomotic recurrence.
CONCLUSION
CTE may be a viable option for the early postsurgical surveillance of recurred disease in CD patients.
Keyword
MeSH Terms
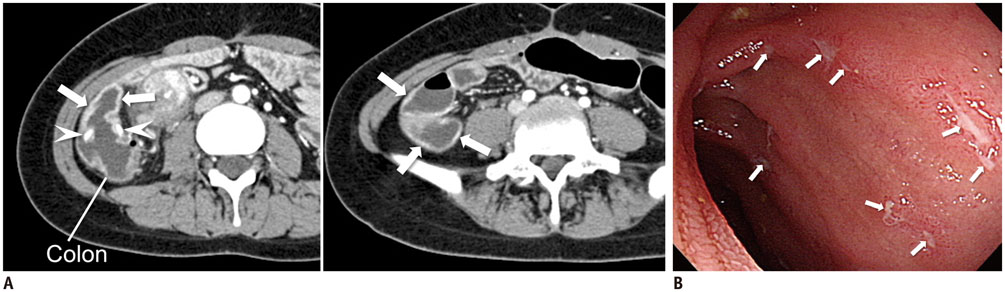
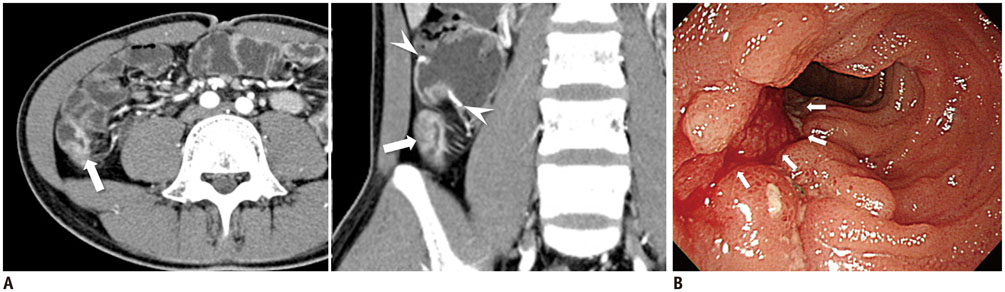
Figure
Cited by 3 articles
-
Preparation, Technique, and Imaging of Computed Tomography/Magnetic Resonance Enterography
Min Ju Kim
Korean J Gastroenterol. 2020;75(2):86-93. doi: 10.4166/kjg.2020.75.2.86.Iodine Quantification on Spectral Detector-Based Dual-Energy CT Enterography: Correlation with Crohn's Disease Activity Index and External Validation
Yeon Soo Kim, Se Hyung Kim, Hwa Sung Ryu, Joon Koo Han
Korean J Radiol. 2018;19(6):1077-1088. doi: 10.3348/kjr.2018.19.6.1077.Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.
Reference
-
1. Hashash JG, Regueiro M. A practical approach to preventing postoperative recurrence in Crohn’s disease. Curr Gastroenterol Rep. 2016; 18:25.2. Yamamoto T. Diagnosis and monitoring of postoperative recurrence in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2015; 9:55–66.3. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990; 99:956–963.4. Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2012; 35:625–633.5. Yang Z, Wu Q, Wu K, Fan D. Management of postoperative Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2014; 8:811–818.6. Nakase H, Keum B, Ye BD, Park SJ, Koo HS, Eun CS. Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:231–239.7. De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015; 385:1406–1417.8. Burke JP, Doherty GA, O’Connell PR. A survey of current practices used to maintain surgically induced remission following intestinal resection for Crohn’s disease. Int J Colorectal Dis. 2013; 28:1073–1079.9. Mao R, Gao X, Zhu ZH, Feng ST, Chen BL, He Y, et al. CT enterography in evaluating postoperative recurrence of Crohn’s disease after ileocolic resection: complementary role to endoscopy. Inflamm Bowel Dis. 2013; 19:977–982.10. Paparo F, Revelli M, Puppo C, Bacigalupo L, Garello I, Garlaschi A, et al. Crohn’s disease recurrence in patients with ileocolic anastomosis: value of computed tomography enterography with water enema. Eur J Radiol. 2013; 82:e434–e440.11. Soyer P, Boudiaf M, Sirol M, Dray X, Aout M, Duchat F, et al. Suspected anastomotic recurrence of Crohn disease after ileocolic resection: evaluation with CT enteroclysis. Radiology. 2010; 254:755–764.12. Minordi LM, Vecchioli A, Poloni G, Guidi L, De Vitis I, Bonomo L. Enteroclysis CT and PEG-CT in patients with previous small-bowel surgical resection for Crohn’s disease: CT findings and correlation with endoscopy. Eur Radiol. 2009; 19:2432–2440.13. Gallego Ojea JC, Echarri Piudo AI, Porta Vila A. [Crohn’s disease: the usefulness of MR enterography in the detection of recurrence after surgery]. Radiologia. 2011; 53:552–559.14. Koilakou S, Sailer J, Peloschek P, Ferlitsch A, Vogelsang H, Miehsler W, et al. Endoscopy and MR enteroclysis: equivalent tools in predicting clinical recurrence in patients with Crohn’s disease after ileocolic resection. Inflamm Bowel Dis. 2010; 16:198–203.15. Sailer J, Peloschek P, Reinisch W, Vogelsang H, Turetschek K, Schima W. Anastomotic recurrence of Crohn’s disease after ileocolic resection: comparison of MR enteroclysis with endoscopy. Eur Radiol. 2008; 18:2512–2521.16. De Cruz P, Bernardi MP, Kamm MA, Allen PB, Prideaux L, Williams J, et al. Postoperative recurrence of Crohn’s disease: impact of endoscopic monitoring and treatment step-up. Colorectal Dis. 2013; 15:187–197.17. Booya F, Akram S, Fletcher JG, Huprich JE, Johnson CD, Fidler JL, et al. CT enterography and fistulizing Crohn’s disease: clinical benefit and radiographic findings. Abdom Imaging. 2009; 34:467–475.18. Bruining DH, Siddiki HA, Fletcher JG, Tremaine WJ, Sandborn WJ, Loftus EV Jr. Prevalence of penetrating disease and extraintestinal manifestations of Crohn’s disease detected with CT enterography. Inflamm Bowel Dis. 2008; 14:1701–1706.19. Park SH, Yang SK, Park SK, Kim JW, Yang DH, Jung KW, et al. Long-term prognosis of Crohn’s disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis. 2014; 20:488–494.20. Qiu Y, Mao R, Chen BL, Li XH, He Y, Zeng ZR, et al. Systematic review with meta-analysis: magnetic resonance enterography vs. computed tomography enterography for evaluating disease activity in small bowel Crohn’s disease. Aliment Pharmacol Ther. 2014; 40:134–146.21. Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003; 228:303–308.22. Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008; 23:1213–1221.23. Simillis C, Yamamoto T, Reese GE, Umegae S, Matsumoto K, Darzi AW, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol. 2008; 103:196–205.24. Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: special situations. J Crohns Colitis. 2010; 4:63–101.25. Huh J, Kim KJ, Park SH, Park SH, Yang SK, Ye BD, et al. Diffusion-weighted MR enterography to monitor bowel inflammation after medical therapy in Crohn’s disease: a prospective longitudinal study. Korean J Radiol. 2017; 18:162–172.26. Choi SH, Kim KW, Lee JY, Kim KJ, Park SH. Diffusion-weighted magnetic resonance enterography for evaluating bowel inflammation in Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2016; 22:669–679.27. Park SH. DWI at MR enterography for evaluating bowel inflammation in Crohn disease. AJR Am J Roentgenol. 2016; 207:40–48.28. Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn's disease. Inflamm Bowel Dis. 2011; 17:1073–1080.29. Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009; 251:751–761.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Study between Axial and Coronal Planes of CT Enterography in Evaluation of Disease Activity and Complications of Crohn Disease
- The role of small bowel endoscopy in small bowel Crohn's disease: when and how?
- Role of Computed Tomography Enterography/Magnetic Resonance Enterography: Is It in Prime Time?
- Computed Tomography Enterography and Magnetic Resonance Enterography in the Diagnosis of Crohn's Disease
- Preparation, Technique, and Imaging of Computed Tomography/Magnetic Resonance Enterography