Yonsei Med J.
2016 Nov;57(6):1412-1419. 10.3349/ymj.2016.57.6.1412.
Antibody to FcεRIα Suppresses Immunoglobulin E Binding to High-Affinity Receptor I in Allergic Inflammation
- Affiliations
-
- 1Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. MHSOHN@yuhs.ac
- 2CRID Center, NeoPharm Co., Ltd., Daejeon, Korea.
- KMID: 2427159
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1412
Abstract
- PURPOSE
High-affinity receptor I (FcεRI) on mast cells and basophils plays a key role in the immunoglobulin E (IgE)-mediated type I hypersensitivity mediated by allergen cross-linking of the specific IgE-FcεRI complex. Thus, prevention of IgE binding to FcεRI on these cells is an effective therapy for allergic disease. We have developed a strategy to disrupt IgE binding to FcεRI using an antibody targeting FcεRIα.
MATERIALS AND METHODS
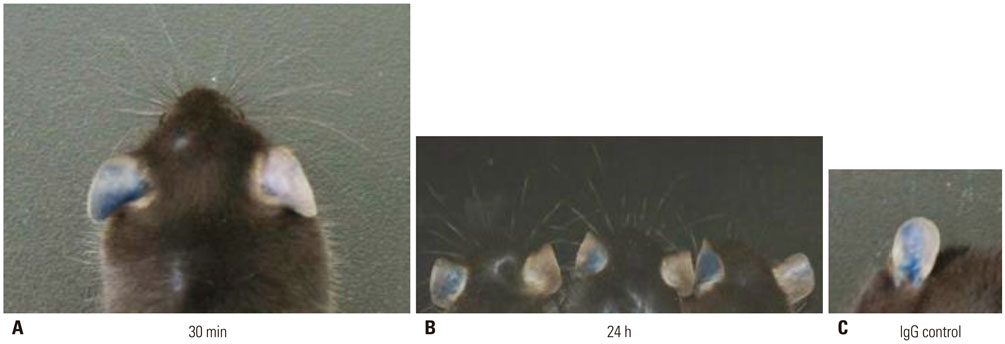
Fab fragment antibodies, which lack the Fc domain, with high affinity and specificity for FcεRIα and effective inhibitory activity against IgE-FcεRI binding were screened. IgE-induced histamine, β-hexosaminidase and Ca2+ release in basophils were determined by ELISA. A B6.Cg-Fcer1a(tm1Knt) Tg(FCER1A)1Bhk/J mouse model of passive cutaneous anaphylaxis (PCA) was used to examine the inhibitory effect of NPB311 on allergic skin inflammation.
RESULTS
NPB311 exhibited high affinity to human FcεRIα (KD=4 nM) and inhibited histamine, β-hexosaminidase and Ca2+ release in a concentration-dependent manner in hFcεRI-expressing cells. In hFcεRIα-expressing mice, dye leakage was higher in the PCA group than in controls, but decreased after NPB311 treatment. NPB311 could form a complex with FcεRIα and inhibit the release of inflammation mediators.
CONCLUSION
Our approach for producing anti-FcεRIα Fab fragment antibody NPB311 may enable clinical application to a therapeutic pathway in IgE/FcεRI-mediated diseases.
Keyword
MeSH Terms
-
Allergens
Animals
Antibodies, Monoclonal/metabolism/*pharmacology
Basophils/*immunology/metabolism
Humans
Hypersensitivity/immunology
Immunoglobulin E/immunology/*metabolism/physiology
Immunoglobulin Fab Fragments/*metabolism
Inflammation/metabolism
Mast Cells
Mice
Receptors, IgE/*immunology/metabolism/physiology
Allergens
Antibodies, Monoclonal
Immunoglobulin Fab Fragments
Receptors, IgE
Immunoglobulin E
Figure
Reference
-
1. Garman SC, Wurzburg BA, Tarchevskaya SS, Kinet JP, Jardetzky TS. Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc epsilonRI alpha. Nature. 2000; 406:259–266.
Article2. Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999; 402:6760 Suppl. B24–B30.3. Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007; 7:365–378.
Article4. Jönsson F, Daëron M. Mast cells and company. Front Immunol. 2012; 3:16.
Article5. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454.
Article6. Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008; 8:205–217.
Article7. Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002; 2:773–786.
Article8. Oettgen HC, Geha RS. IgE regulation and roles in asthma pathogenesis. J Allergy Clin Immunol. 2001; 107:429–440.
Article9. Wu LC, Scheerens H. Targeting IgE production in mice and humans. Curr Opin Immunol. 2014; 31:8–15.
Article10. Bax HJ, Keeble AH, Gould HJ. Cytokinergic IgE action in mast cell activation. Front Immunol. 2012; 3:229.
Article11. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010; 125:2 Suppl 2. S73–S80.
Article12. Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness-a murine model. Allergy. 1999; 54:297–305.
Article13. Arshad SH, Holgate S. The role of IgE in allergen-induced inflammation and the potential for intervention with a humanized monoclonal anti-IgE antibody. Clin Exp Allergy. 2001; 31:1344–1351.
Article14. Corren J, Casale TB, Lanier B, Buhl R, Holgate S, Jimenez P. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009; 39:788–797.
Article15. MacGlashan D Jr. Therapeutic efficacy of omalizumab. J Allergy Clin Immunol. 2009; 123:114–115.
Article16. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006; 354:2689–2695.
Article17. Ra C, Jouvin MH, Kinet JP. Complete structure of the mouse mast cell receptor for IgE (Fc epsilon RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J Biol Chem. 1989; 264:15323–15327.
Article18. Ausländer D, Eggerschwiler B, Kemmer C, Geering B, Ausländer S, Fussenegger M. A designer cell-based histamine-specific human allergy profiler. Nat Commun. 2014; 5:4408.
Article19. Hochhaus G, Brookman L, Fox H, Johnson C, Matthews J, Ren S, et al. Pharmacodynamics of omalizumab: implications for optimised dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin. 2003; 19:491–498.
Article20. Lee YH, Yoon SJ, Kim EJ, Kim YA, Seo HY, Oh IH. Economic burden of asthma in Korea. Allergy Asthma Proc. 2011; 32:35–40.
Article21. Levy AN, García A, García-Agua Soler N, Sanjuan MV. Cost-effectiveness of omalizumab in severe persistent asthma in Spain: a real-life perspective. J Asthma. 2015; 52:205–210.
Article22. Chu SY, Horton HM, Pong E, Leung IW, Chen H, Nguyen DH, et al. Reduction of total IgE by targeted coengagement of IgE B-cell receptor and FcεRIIb with Fc-engineered antibody. J Allergy Clin Immunol. 2012; 129:1102–1115.
Article23. Landolina N, Levi-Schaffer F. Monoclonal antibodies: the new magic bullets for allergy: IUPHAR Review 17. Br J Pharmacol. 2016; 173:793–803.
Article24. Han DK, Kim MK, Yoo JE, Choi SY, Kwon BC, Sohn MH, et al. Food sensitization in infants and young children with atopic dermatitis. Yonsei Med J. 2004; 45:803–809.
Article25. Sheinkopf LE, Rafi AW, Do LT, Katz RM, Klaustermeyer WB. Efficacy of omalizumab in the treatment of atopic dermatitis: a pilot study. Allergy Asthma Proc. 2008; 29:530–537.
Article26. Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course - a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010; 8:990–998.
Article27. Ozdemir D, Dagdelen S, Erbas T. Systemic mastocytosis. Am J Med Sci. 2011; 342:409–415.
Article28. Jevševar S, Kusterle M, Kenig M. PEGylation of antibody fragments for half-life extension. Methods Mol Biol. 2012; 901:233–246.
Article29. Flanagan RJ, Jones AL. Fab antibody fragments: some applications in clinical toxicology. Drug Saf. 2004; 27:1115–1133.30. Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005; 23:1126–1136.
Article31. Ko YJ, Kim HH, Kim EJ, Katakura Y, Lee WS, Kim GS, et al. Piceatannol inhibits mast cell-mediated allergic inflammation. Int J Mol Med. 2013; 31:951–958.
Article32. Matsuoka D, Mizutani N, Sae-Wong C, Yoshino S. Allergen-specific regulation of allergic rhinitis in mice by intranasal exposure to IgG1 monoclonal antibody Fab fragments against pathogenic allergen. Immunol Lett. 2014; 161:149–156.
Article33. Yoshino S, Mizutani N, Matsuoka D, Sae-Wong C. Intratracheal exposure to Fab fragments of an allergen-specific monoclonal antibody regulates asthmatic responses in mice. Immunology. 2014; 141:617–627.
Article34. Hacha J, Tomlinson K, Maertens L, Paulissen G, Rocks N, Foidart JM, et al. Nebulized anti-IL-13 monoclonal antibody Fab' fragment reduces allergen-induced asthma. Am J Respir Cell Mol Biol. 2012; 47:709–717.
Article35. Yalcin AD, Bisgin A, Gorczynski RM. IL-8, IL-10, TGF-β, and GCSF levels were increased in severe persistent allergic asthma patients with the anti-IgE treatment. Mediators Inflamm. 2012; 2012:720976.36. Nelson AL. Antibody fragments: hope and hype. MAbs. 2010; 2:77–83.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The binding affinity of glucocorticoid receptor in the peripheral blood mononuclear cells from patients with steroid-resistant and steroid-responsive asthma
- Effect of Amino Acid Polymorphisms of House Dust Mite Der p 2 Variants on Allergic Sensitization
- Purification of Opioid Receptor in the Presence of Sodium Ion
- Inhibition of Murine Allergic Response by Monoclonal Interleukin-4 Receptor Antibody
- Functions of Siglecs in Allergic Inflammation