Allergy Asthma Immunol Res.
2019 Jan;11(1):90-103. 10.4168/aair.2019.11.1.90.
Prevalence and Clinical Features of Drug Reactions With Eosinophilia and Systemic Symptoms Syndrome Caused by Antituberculosis Drugs: A Retrospective Cohort Study
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. sanghakim@yonsei.ac.kr
- KMID: 2426788
- DOI: http://doi.org/10.4168/aair.2019.11.1.90
Abstract
- PURPOSE
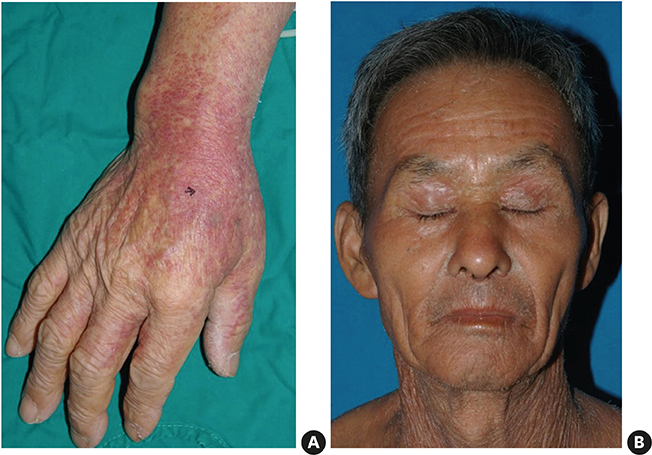
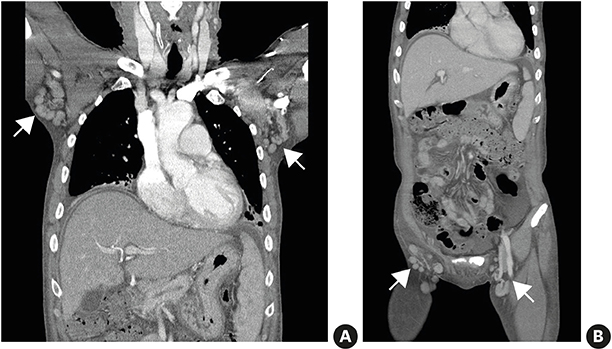
Although there have been reported cases of drug reactions with eosinophilia and systemic symptoms (DRESS) syndrome caused by antituberculosis drugs, there has been no research to examine its prevalence. This study assessed the prevalence and clinical characteristics of DRESS syndrome caused by antituberculosis drugs.
METHODS
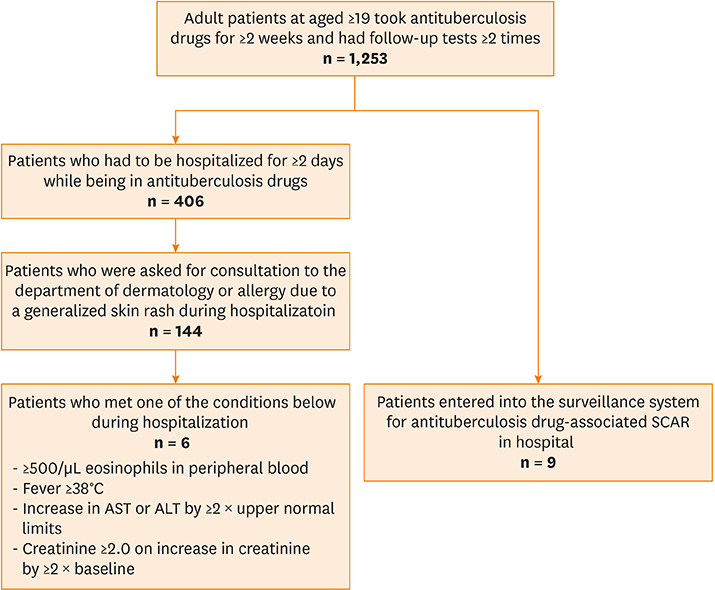
The electronic medical records of a cohort consisting of adult patients diagnosed with tuberculosis between July 2006 and June 2010 were reviewed and retrospectively inspected. We searched the surveillance system for adverse drug reactions and the electronic medical records to identify patients who reported severe cutaneous adverse reactions to antituberculosis drugs. These patients were then re-assessed using a European Registry of Severe Cutaneous Adverse Reactions to Drugs and Collection of Biological Samples (RegiSCAR) scoring system. Clinical characteristics, including the symptoms and latency of DRESS syndrome, the therapeutic dosage and period of steroids, and the final duration of tuberculosis therapy, were examined.
RESULTS
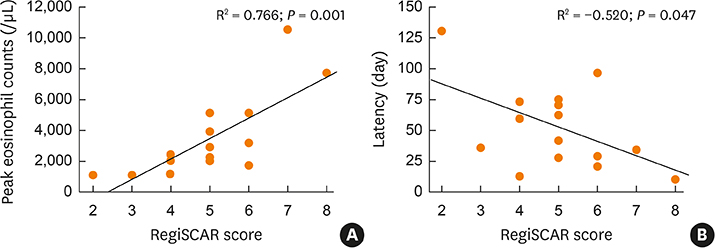
Of the 1,253 adult patients with tuberculosis receiving antituberculosis drugs, 15 were identified as potential cases of DRESS syndrome (prevalence of 1.2%). Ethambutol was the most frequently used drug (53.5%), followed by rifampicin (26.7%), pyrazinamide (20.0%), streptomycin (13.3%), and isoniazid (6.7%). The median latency after day 1 of antituberculosis medication was 42 days. The median daily dose of steroids, expressed in prednisone-equivalent units, was 33-mg/day, and the median dosing period was 14 days. The duration of tuberculosis treatment was 76 days longer than the standard treatment period of 180 days. There was a significant difference in the peak eosinophil counts of DRESS syndrome patients according to RegiSCAR scores. Moreover, there was a significant quantitative correlation between the RegiSCAR score and peak eosinophil count. A negative correlation was also found between the RegiSCAR score and latency.
CONCLUSIONS
This study confirmed the prevalence of DRESS syndrome in a cohort of adult patients with tuberculosis.
MeSH Terms
-
Adult
Cohort Studies*
Drug Hypersensitivity Syndrome
Drug-Related Side Effects and Adverse Reactions
Electronic Health Records
Eosinophilia*
Eosinophils
Ethambutol
Humans
Isoniazid
Prevalence*
Pyrazinamide
Retrospective Studies*
Rifampin
Steroids
Streptomycin
Tuberculosis
Ethambutol
Isoniazid
Pyrazinamide
Rifampin
Steroids
Streptomycin
Figure
Cited by 1 articles
-
Severe Cutaneous Adverse Reactions in Korean Pediatric Patients: A Study From the Korea SCAR Registry
Hea Lin Oh, Dong Yoon Kang, Hye-Ryun Kang, Sujeong Kim, Young-Il Koh, Sae Hoon Kim, Min-Hye Kim, Dong In Suh,
Allergy Asthma Immunol Res. 2019;11(2):241-253. doi: 10.4168/aair.2019.11.2.241.
Reference
-
1. Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg. 1996; 15:250–257.
Article2. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. Clinical perspectives. J Am Acad Dermatol. 2013; 68:639.e1–639.e14.3. Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: a literature review. Am J Med. 2011; 124:588–597.
Article4. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003; 167:603–662.5. Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996; 9:2026–2030.
Article6. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003; 167:1472–1477.
Article7. Breen RA, Miller RF, Gorsuch T, Smith CJ, Schwenk A, Holmes W, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax. 2006; 61:791–794.
Article8. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007; 156:609–611.
Article9. Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013; 169:1071–1080.
Article10. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000; 356:1255–1259.
Article11. Karlin E, Phillips E. Genotyping for severe drug hypersensitivity. Curr Allergy Asthma Rep. 2014; 14:418.
Article12. Park HW, Kim SH, Chang YS, Kim SH, Jee YK, Lee AY, et al. The Fas signaling pathway is a common genetic risk factor for severe cutaneous drug adverse reactions across diverse drugs. Allergy Asthma Immunol Res. 2018; 10:555–561.
Article13. Kim SH, Lee SK, Kim SH, Park HW, Chang YS, Lee KW, et al. Antituberculosis drug-induced hypersensitivity syndrome and its association with human leukocyte antigen. Tuberculosis (Edinb). 2013; 93:270–274.
Article14. Allouchery M, Logerot S, Cottin J, Pralong P, Villier C, Ben Saïd B, et al. Antituberculosis drug-associated DRESS: a case series. J Allergy Clin Immunol Pract. 2018; 6:1373–1380.
Article15. Lee JH, Park HK, Heo J, Kim TO, Kim GH, Kang DH, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008; 23:521–525.
Article16. Lee SW, Yoon NB, Park SM, Lee SM, Um SJ, Lee SK, et al. Antituberculosis drug-induced drug rash with eosinophilia and systemic symptoms syndrome confirmed by patch testing. J Investig Allergol Clin Immunol. 2010; 20:631–632.17. Ogawa K, Morito H, Kobayashi N, Fukumoto T, Asada H. Case of drug-induced hypersensitivity syndrome involving multiple-drug hypersensitivity. J Dermatol. 2012; 39:945–946.
Article18. Jung ES, Choi B, Choi HS, Kim BH, Ha M, Shin D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome induced by ethambutol and rifampin. Infect Chemother. 2012; 44:197–200.
Article19. Shebe K, Ngwanya MR, Gantsho N, Lehloenya RJ. Severe recurrence of drug rash with eosinophilia and systemic symptoms syndrome secondary to rifampicin patch testing in a human immunodeficiency virus-infected man. Contact Dermat. 2014; 70:125–127.
Article20. Lee JY, Seol YJ, Shin DW, Kim DY, Chun HW, Kim BY, et al. A case of the drug reaction with eosinophilia and systemic symptom (DRESS) following isoniazid treatment. Tuberc Respir Dis (Seoul). 2015; 78:27–30.
Article21. Zhang SN, He QX, Yang NB, Ni SL, Lu MQ. Isoniazid-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome presenting as acute eosinophilic myocarditis. Intern Med. 2015; 54:1227–1230.
Article22. Kim H, Bang ES, Lim SK, Lee JM. DRESS syndrome and acute generalized exanthematous pustulosis induced by antituberculosis medications and moxifloxacin: case report . Int J Clin Pharmacol Ther. 2016; 54:808–815.
Article23. Yoshioka Y, Hanafusa T, Namiki T, Nojima K, Amano M, Tokoro S, et al. Drug-induced hypersensitivity syndrome by ethambutol: a case report. J Dermatol. 2016; 43:971–972.
Article24. Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009; 9:153–161.
Article25. Lehloenya RJ, Dheda K. Cutaneous adverse drug reactions to anti-tuberculosis drugs: state of the art and into the future. Expert Rev Anti Infect Ther. 2012; 10:475–486.
Article26. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Proposed by the Working party of the ESCD for the study of skin testing in investigating cutaneous adverse drug reactions. Contact Dermat. 2001; 45:321–328.27. Elzagallaai AA, Knowles SR, Rieder MJ, Bend JR, Shear NH, Koren G. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009; 32:391–408.28. Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000; 136:323–327.
Article29. Gentile I, Talamo M, Borgia G. Is the drug-induced hypersensitivity syndrome (DIHS) due to human herpesvirus 6 infection or to allergy-mediated viral reactivation? Report of a case and literature review. BMC Infect Dis. 2010; 10:49.
Article30. Hirahara K, Kano Y, Mitsuyama Y, Takahashi R, Kimishima M, Shiohara T. Differences in immunological alterations and underlying viral infections in two well-defined severe drug eruptions. Clin Exp Dermatol. 2010; 35:863–868.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cutaneous adverse drug reactions
- An Overview of Multiple Drug Hypersensitivity Syndrome
- Recent advances of pharmacogenomics in severe cutaneous adverse reactions: immune and nonimmune mechanisms
- Drug Reaction with Eosinophilia and Systemic Symptom Syndrome Due to Everolimus: A Case Report
- Piperacillin/Tazobactam-Associated Hypersensitivity Syndrome with Overlapping Features of Acute Generalized Exanthematous Pustulosis and Drug-Related Rash with Eosinophilia and Systemic Symptoms Syndrome