Obstet Gynecol Sci.
2018 Sep;61(5):584-589. 10.5468/ogs.2018.61.5.584.
Effect of tibolone on the survival of early stage cervical adenocarcinoma patients
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea. leekwbm@naver.com
- KMID: 2426023
- DOI: http://doi.org/10.5468/ogs.2018.61.5.584
Abstract
OBJECTIVE
Gynecologic oncologists are uncertain about the safety of tibolone application in cervical adenocarcinoma (AC) patients. This study examined the possible adverse effects of tibolone on the survival of cervical AC patients.
METHODS
Medical records of 70 cervical AC patients with International Federation of Gynecology and Obstetrics stages IA to IB were reviewed. A bilateral salpingo-oophorectomy was performed in all patients, and survival outcomes between tibolone users (n=38) and non-users (n=32) were compared.
RESULTS
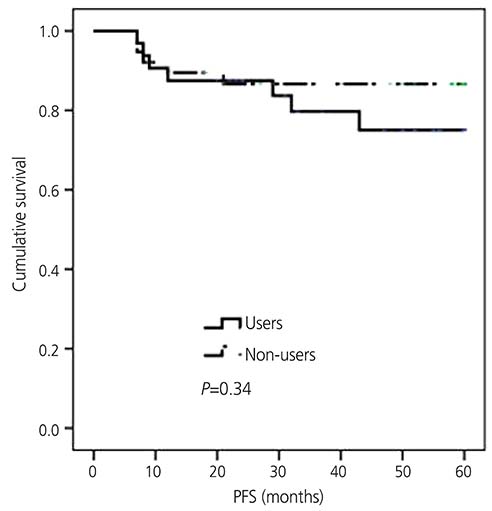
A comparison of the tibolone users with non-users revealed similar clinicopathological variables. Progression-free survival (P=0.34) and overall survival (P=0.22) were similar in the users and non-users. The risks of progression (hazard ratio [HR], 1.71; 95% confidence interval [CI], 0.46-6.37; P=0.43) and death (HR, 1.59; 95% CI, 0.06-45.66; P=0.79) were also similar in both groups.
CONCLUSION
Tibolone has no adverse effect on the survival of cervical AC patients and can be administered safely to this population. These findings may be helpful in improving the quality of life of cervical AC patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
Article2. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–450.
Article3. Fujiwara H, Yokota H, Monk B, Treilleux I, Devouassoux-Shisheboran M, Davis A, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for cervical adenocarcinoma. Int J Gynecol Cancer. 2014; 24:S96–101.
Article4. MacLennan A, Lester S, Moore V. Oral estrogen replacement therapy versus placebo for hot flushes: a systematic review. Climacteric. 2001; 4:58–74.
Article5. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002; 288:321–333.6. Irie T, Kigawa J, Minagawa Y, Itamochi H, Sato S, Akeshima R, et al. Prognosis and clinicopathological characteristics of Ib-IIb adenocarcinoma of the uterine cervix in patients who have had radical hysterectomy. Eur J Surg Oncol. 2000; 26:464–467.
Article7. Masood S, Rhatigan RM, Wilkinson EW, Barwick KW, Wilson WJ. Expression and prognostic significance of estrogen and progesterone receptors in adenocarcinoma of the uterine cervix. An immunocytochemical study. Cancer. 1993; 72:511–518.
Article8. Bodner K, Laubichler P, Kimberger O, Czerwenka K, Zeillinger R, Bodner-Adler B. Oestrogen and progesterone receptor expression in patients with adenocarcinoma of the uterine cervix and correlation with various clinicopathological parameters. Anticancer Res. 2010; 30:1341–1345.9. Parazzini F, La Vecchia C. Epidemiology of adenocarcinoma of the cervix. Gynecol Oncol. 1990; 39:40–46.
Article10. Everhov AH, Nyberg T, Bergmark K, Citarella A, Rådestad AF, Hirschberg AL, et al. Hormone therapy after uterine cervical cancer treatment: a Swedish population-based study. Menopause. 2015; 22:633–639.11. O'Donnell RL, Clement KM, Edmondson RJ. Hormone replacement therapy after treatment for a gynaecological malignancy. Curr Opin Obstet Gynecol. 2016; 28:32–41.12. Biglia N, Maffei S, Lello S, Nappi RE. Tibolone in postmenopausal women: a review based on recent randomised controlled clinical trials. Gynecol Endocrinol. 2010; 26:804–814.
Article13. Lee KB, Lee JM, Lee JK, Cho CH. Endometrial cancer patients and tibolone: a matched case-control study. Maturitas. 2006; 55:264–269.
Article14. Lee KB, Lee JM, Yoon JH, Park CY. The safety of tibolone in epithelial ovarian cancer patients. Maturitas. 2006; 55:156–161.
Article15. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol. 1987; 26:169–177.
Article16. Lacey JV Jr, Brinton LA, Barnes WA, Gravitt PE, Greenberg MD, Hadjimichael OC, et al. Use of hormone replacement therapy and adenocarcinomas and squamous cell carcinomas of the uterine cervix. Gynecol Oncol. 2000; 77:149–154.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of patients in whom Endometrial adenocarcinoma was diagnosed while on therapy with Tibolone
- Ovarian Metastasis from Stage IB Cervical Adenocarcinoma: A Case Report
- Tibolone and Breast Cancer
- A case of extremely early cervical adenocarcinoma diagnosed only by endocervical curettage with macroscopic pelvic lymph node metastases
- Clinicopathologic characteristics and prognostic factors in adenocarcinoma of the uterine cervix