J Clin Neurol.
2012 Jun;8(2):89-99.
Sensitization of the Trigeminovascular Pathway: Perspective and Implications to Migraine Pathophysiology
- Affiliations
-
- 1Department of Anaesthesia Neuroscience, Comprehensive Headache Center, Harvard Medical School, Boston, MA, USA. rburstei@bidmc.harvard.edu
- 2Departments of Anesthesia and Critical Care, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Abstract
- Migraine headache is commonly associated with signs of exaggerated intracranial and extracranial mechanical sensitivities. Patients exhibiting signs of intracranial hypersensitivity testify that their headache throbs and that mundane physical activities that increase intracranial pressure (such as bending over or coughing) intensify the pain. Patients exhibiting signs of extracranial hypersensitivity testify that during migraine their facial skin hurts in response to otherwise innocuous activities such as combing, shaving, letting water run over their face in the shower, or wearing glasses or earrings (termed here cephalic cutaneous allodynia). Such patients often testify that during migraine their bodily skin is hypersensitive and that wearing tight cloth, bracelets, rings, necklaces and socks or using a heavy blanket can be uncomfortable and/or painful (termed her extracephalic cutaneous allodynia). This review summarizes the evidence that support the view that activation of the trigeminovascular pathway contribute to the headache phase of a migraine attack, that the development of throbbing in the initial phase of migraine is mediated by sensitization of peripheral trigeminovascular neurons that innervate the meninges, that the development of cephalic allodynia is propelled by sensitization of second-order trigeminovascular neurons in the spinal trigeminal nucleus which receive converging sensory input from the meninges as well as from the scalp and facial skin, and that the development of extracephalic allodynia is mediated by sensitization of third-order trigeminovascular neurons in the posterior thalamic nuclei which receive converging sensory input from the meninges, facial and body skin.
Keyword
MeSH Terms
Figure
Reference
-
1. Anthony M. The treatment of migraine--old methods, new ideas. Aust Fam Physician. 1993; 22:1434–1435. 1438–1439. 1442–1443. PMID: 8379882.2. Blau JN, Dexter SL. The site of pain origin during migraine attacks. Cephalalgia. 1981; 1:143–147. PMID: 7346182.
Article3. Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991; 44:1147–1157. PMID: 1941010.
Article4. Wolff HG. Headache and Other Head Pain. 1963. 2nd ed. New York: Oxford University Press.5. Wolff HG, Tunis MM, Goodell H. Studies on headache; evidence of damage and changes in pain sensitivity in subjects with vascular headaches of the migraine type. AMA Arch Intern Med. 1953; 92:478–484. PMID: 13091465.6. Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev. 1993; 5:159–177. PMID: 8217498.7. Zwetsloot CP, Caekebeke JF, Jansen JC, Odink J, Ferrari MD. Blood flow velocity changes in migraine attacks--a transcranial Doppler study. Cephalalgia. 1991; 11:103–107. PMID: 1860130.
Article8. Ray BS, Wolff HG. Experimental studies on headache. Pain-sensitive structures of the head and their significance in headache. Arch Surg. 1940; 41:813–856.9. Penfield W, McNaughton F. Dural headache and innervation of the dura mater. Arch Neurol Psychiatry. 1940; 44:43–75.
Article10. Keller JT, Saunders MC, Beduk A, Jollis JG. Innervation of the posterior fossa dura of the cat. Brain Res Bull. 1985; 14:97–102. PMID: 3872702.
Article11. Andres KH, von Düring M, Muszynski K, Schmidt RF. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl). 1987; 175:289–301. PMID: 3826655.
Article12. Mayberg M, Langer RS, Zervas NT, Moskowitz MA. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981; 213:228–230. PMID: 6166046.
Article13. Uddman R, Edvinsson L, Ekman R, Kingman T, McCulloch J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett. 1985; 62:131–136. PMID: 2415882.
Article14. Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol. 1991; 309:515–534. PMID: 1717522.
Article15. Strassman A, Mason P, Moskowitz M, Maciewicz R. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res. 1986; 379:242–250. PMID: 3742223.
Article16. Davis KD, Dostrovsky JO. Activation of trigeminal brain-stem nociceptive neurons by dural artery stimulation. Pain. 1986; 25:395–401. PMID: 3748590.
Article17. Goadsby PJ, Zagami AS. Stimulation of the superior sagittal sinus increases metabolic activity and blood flow in certain regions of the brainstem and upper cervical spinal cord of the cat. Brain. 1991; 114:1001–1011. PMID: 2043937.
Article18. Kaube H, Keay KA, Hoskin KL, Bandler R, Goadsby PJ. Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical spinal cord following stimulation of the superior sagittal sinus in the cat. Brain Res. 1993; 629:95–102. PMID: 8287282.
Article19. Zagami AS, Goadsby PJ, Edvinsson L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides. 1990; 16:69–75. PMID: 2250767.
Article20. Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994; 14:3725–3735. PMID: 8207485.
Article21. Davis KD, Dostrovsky JO. Responses of feline trigeminal spinal tract nucleus neurons to stimulation of the middle meningeal artery and sagittal sinus. J Neurophysiol. 1988; 59:648–666. PMID: 3351579.
Article22. Benjamin L, Levy MJ, Lasalandra MP, Knight YE, Akerman S, Classey JD, et al. Hypothalamic activation after stimulation of the superior sagittal sinus in the cat: a Fos study. Neurobiol Dis. 2004; 16:500–505. PMID: 15262261.
Article23. Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc Natl Acad Sci U S A. 2001; 98:9930–9935. PMID: 11504950.
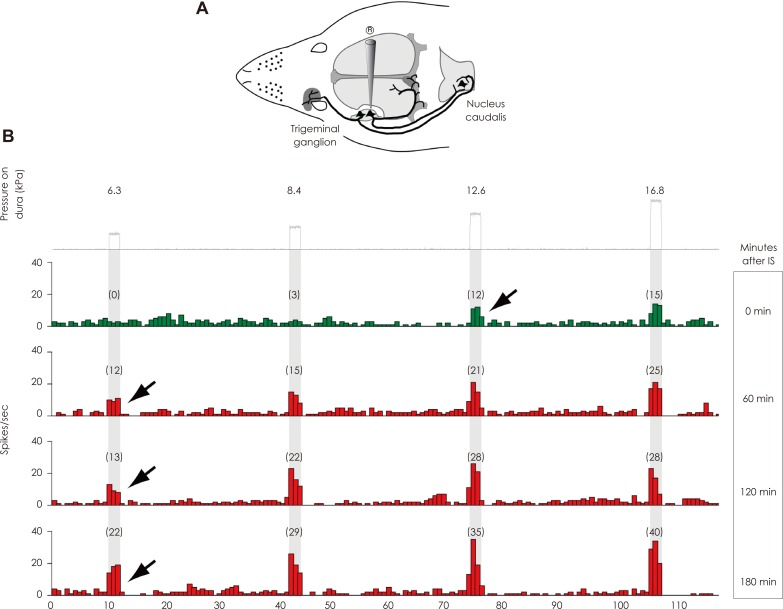
Article24. Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol. 2002; 88:3021–3031. PMID: 12466427.
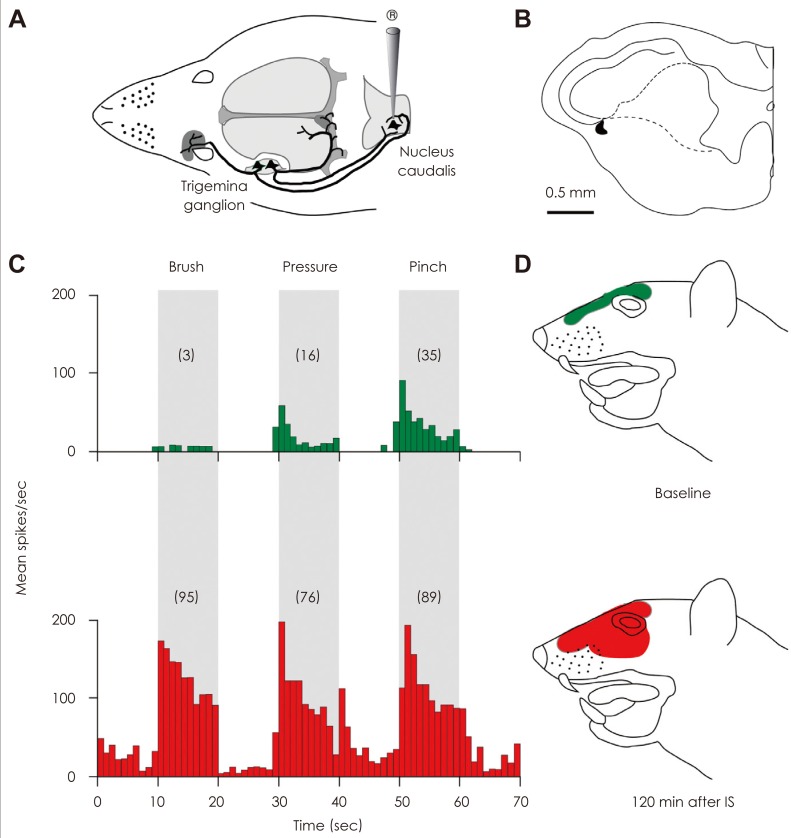
Article25. Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998; 79:964–982. PMID: 9463456.
Article26. Yamamura H, Malick A, Chamberlin NL, Burstein R. Cardiovascular and neuronal responses to head stimulation reflect central sensitization and cutaneous allodynia in a rat model of migraine. J Neurophysiol. 1999; 81:479–493. PMID: 10036252.27. Zagami AS, Lambert GA. Stimulation of cranial vessels excites nociceptive neurones in several thalamic nuclei of the cat. Exp Brain Res. 1990; 81:552–566. PMID: 2226688.
Article28. Davis KD, Dostrovsky JO. Properties of feline thalamic neurons activated by stimulation of the middle meningeal artery and sagittal sinus. Brain Res. 1988; 454:89–100. PMID: 3409027.
Article29. Goadsby PJ, Hoskin KL. Inhibition of trigeminal neurons by intravenous administration of the serotonin (5HT)1B/D receptor agonist zolmitriptan (311C90): are brain stem sites therapeutic target in migraine? Pain. 1996; 67:355–359. PMID: 8951929.30. Hoskin KL, Kaube H, Goadsby PJ. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiological study. Brain. 1996; 119:249–256. PMID: 8624686.31. Lambert GA, Lowy AJ, Boers PM, Angus-Leppan H, Zagami AS. The spinal cord processing of input from the superior sagittal sinus: pathway and modulation by ergot alkaloids. Brain Res. 1992; 597:321–330. PMID: 1473003.
Article32. Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996; 384:560–564. PMID: 8955268.
Article33. Beck PW, Handwerker HO. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974; 347:209–222. PMID: 4857072.34. Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. J Neurophysiol. 1993; 69:1071–1081. PMID: 8492149.
Article35. Mizumura K, Sato J, Kumazawa T. Effects of prostaglandins and other putative chemical intermediaries on the activity of canine testicular polymodal receptors studied in vitro. Pflugers Arch. 1987; 408:565–572. PMID: 2439985.
Article36. Neugebauer V, Schaible HG, Schmidt RF. Sensitization of articular afferents to mechanical stimuli by bradykinin. Pflugers Arch. 1989; 415:330–335. PMID: 2622760.
Article37. Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992; 12:86–95. PMID: 1309578.
Article38. Armstrong D, Jepson JB, Keele CA, Stewart JW. Pain-producing substance in human inflammatory exudates and plasma. J Physiol. 1957; 135:350–370. PMID: 13406745.
Article39. Guzman F, Braun C, Lim RK. Visceral pain and the pseudaffective response to intra-arterial injection of bradykinin and other algesic agents. Arch Int Pharmacodyn Ther. 1962; 136:353–384. PMID: 13903244.40. Hollander W, Michelson AL, Wilkins RW. Serotonin and antiserotonins. I. Their circulatory, respiratory, and renal effects in man. Circulation. 1957; 16:246–255. PMID: 13447168.41. Sicuteri F. Vasoneuractive substances and their implication in vascular pain. Res Clin Stud Headache. 1967; 1:6–45.42. Liveing E. On Megrim, Sick-Headache, and Some Allied Disorders: A Contribution to the Pathology of Nerve-Storms. 1873. London: J. and A. Churchill.43. Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatry. 1960; 23:23–32. PMID: 14444681.
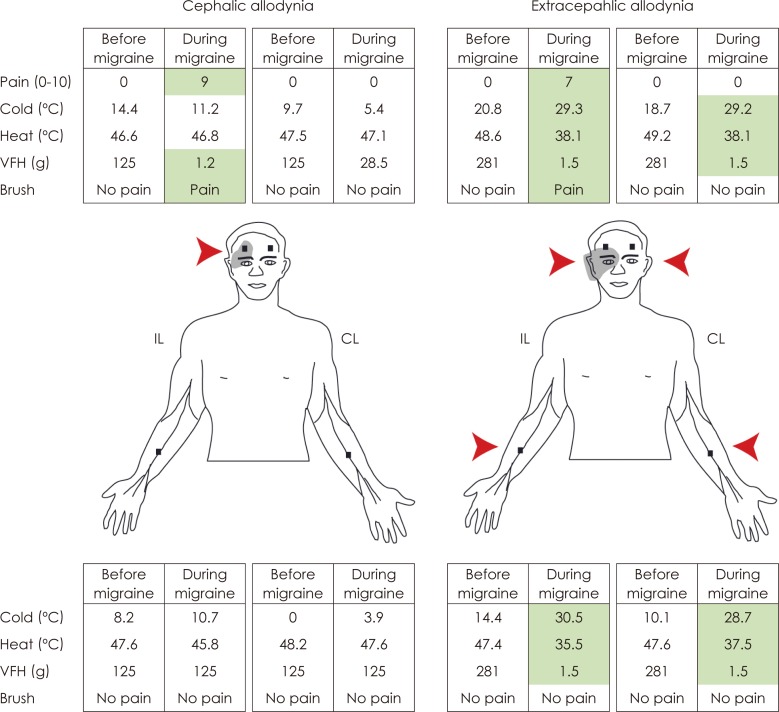
Article44. Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000; 47:614–624. PMID: 10805332.
Article45. Craig AD, Dostrovsky JO. Thermoreceptive lamina I trigeminothalamic neurons project to the nucleus submedius in the cat. Exp Brain Res. 1991; 85:470–474. PMID: 1716595.
Article46. Strassman AM, Potrebic S, Maciewicz RJ. Anatomical properties of brainstem trigeminal neurons that respond to electrical stimulation of dural blood vessels. J Comp Neurol. 1994; 346:349–365. PMID: 7995855.
Article47. Ebersberger A, Ringkamp M, Reeh PW, Handwerker HO. Recordings from brain stem neurons responding to chemical stimulation of the subarachnoid space. J Neurophysiol. 1997; 77:3122–3133. PMID: 9212262.
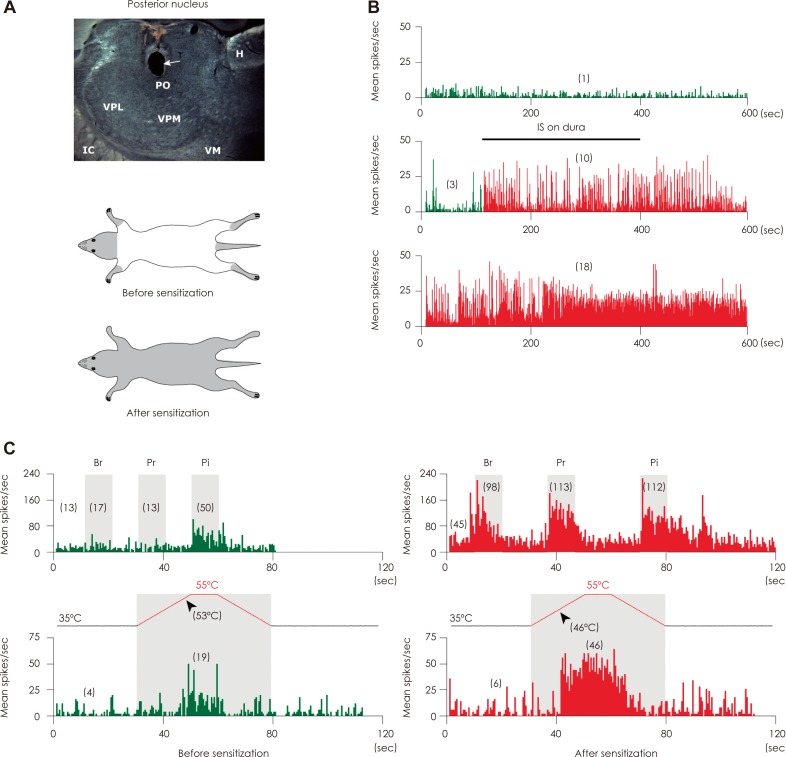
Article48. Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010; 68:81–91. PMID: 20582997.
Article49. Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003; 26:696–705. PMID: 14624855.
Article50. Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004; 55:27–36. PMID: 14705109.
Article51. Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004; 55:19–26. PMID: 14705108.
Article52. Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans. J Neurosci. 2003; 23:10988–10997. PMID: 14645495.
Article53. Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004; 101:4274–4279. PMID: 15016917.54. Mathew NT. Early intervention with almotriptan improves sustained pain-free response in acute migraine. Headache. 2003; 43:1075–1079. PMID: 14629242.
Article56. Weiller C, May A, Limmroth V, Jüptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995; 1:658–660. PMID: 7585147.
Article57. Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001; 41:629–637. PMID: 11554950.
Article58. Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002; 25:319–325. PMID: 12086751.
Article59. Raskin NH, Hosobuchi Y, Lamb S. Headache may arise from perturbation of brain. Headache. 1987; 27:416–420. PMID: 3667258.
Article60. Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001; 357:1016–1017. PMID: 11293599.
Article61. Schaller B, Baumann A. Headache after removal of vestibular schwannoma via the retrosigmoid approach: a long-term follow-up-study. Otolaryngol Head Neck Surg. 2003; 128:387–395. PMID: 12646842.
Article62. Harner SG, Beatty CW, Ebersold MJ. Headache after acoustic neuroma excision. Am J Otol. 1993; 14:552–555. PMID: 8296857.63. Gökalp HZ, Arasil E, Erdogan A, Egemen N, Deda H, Cerçi A. Tentorial meningiomas. Neurosurgery. 1995; 36:46–51. discussion 51. PMID: 7708167.
Article64. Kaur A, Selwa L, Fromes G, Ross DA. Persistent headache after supratentorial craniotomy. Neurosurgery. 2000; 47:633–636. PMID: 10981750.
Article65. Gee JR, Ishaq Y, Vijayan N. Postcraniotomy headache. Headache. 2003; 43:276–278. PMID: 12603648.
Article66. Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, et al. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002; 16:158–168. PMID: 11969326.
Article67. Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999; 83:459–470. PMID: 10568854.
Article68. Hsieh JC, Ståhle-Bäckdahl M, Hägermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996; 64:303–314. PMID: 8740608.
Article69. Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, et al. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998; 121:931–947. PMID: 9619195.
Article70. Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000; 53:95–104. PMID: 11033213.
Article71. Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977; 197:183–186. PMID: 301658.
Article72. Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978; 4:451–462. PMID: 216303.
Article73. Holstege G, Kuypers HG. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res. 1982; 57:145–175. PMID: 7156396.
Article74. Martin GF, Vertes RP, Waltzer R. Spinal projections of the gigantocellular reticular formation in the rat. Evidence for projections from different areas to laminae I and II and lamina IX. Exp Brain Res. 1985; 58:154–162. PMID: 3987846.
Article75. Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995; 74:1742–1759. PMID: 8989409.
Article76. Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984; 16:157–168. PMID: 6206779.
Article77. Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988; 24:739–768. PMID: 3288903.
Article78. Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci. 1987; 7:4129–4136. PMID: 3694267.
Article79. Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991; 44:97–112. PMID: 1771000.
Article80. Buzzi MG, Moskowitz MA. The antimigraine drug, sumatriptan (GR 43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. Br J Pharmacol. 1990; 99:202–206. PMID: 2158835.81. Buzzi MG, Sakas DE, Moskowitz MA. Indomethacin and acetylsalicylic acid block neurogenic plasma protein extravasation in rat dura mater. Eur J Pharmacol. 1989; 165:251–258. PMID: 2776831.82. Leão AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944; 7:359–390.83. Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994; 117:199–210. PMID: 7908596.
Article84. Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001; 98:4687–4692. PMID: 11287655.
Article85. Bowyer SM, Aurora KS, Moran JE, Tepley N, Welch KM. Magnetoencephalographic fields from patients with spontaneous and induced migraine aura. Ann Neurol. 2001; 50:582–587. PMID: 11706963.
Article86. James MF, Smith JM, Boniface SJ, Huang CL, Leslie RA. Cortical spreading depression and migraine: new insights from imaging? Trends Neurosci. 2001; 24:266–271. PMID: 11311378.
Article87. Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011; 69:855–865. PMID: 21416489.
Article88. Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010; 30:8807–8814. PMID: 20592202.
Article89. Brinley FJ Jr, Kandel ER, Marshall WH. Potassium outflux from rabbit cortex during spreading depression. J Neurophysiol. 1960; 23:246–256. PMID: 13804482.
Article90. Rapoport SI, Marshall WH. Measurement of Cortical Ph in Spreading Cortical Depression. Am J Physiol. 1964; 206:1177–1180. PMID: 14208962.
Article91. Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002; 8:136–142. PMID: 11821897.
Article92. Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009; 515:331–348. PMID: 19425099.
Article93. Foley KA, Cady R, Martin V, Adelman J, Diamond M, Bell CF, et al. Treating early versus treating mild: timing of migraine prescription medications among patients with diagnosed migraine. Headache. 2005; 45:538–545. PMID: 15953272.
Article94. Jakubowski M, Levy D, Goor-Aryeh I, Collins B, Bajwa Z, Burstein R. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache. 2005; 45:850–861. PMID: 15985101.
Article95. Jakubowski M, Levy D, Kainz V, Zhang XC, Kosaras B, Burstein R. Sensitization of central trigeminovascular neurons: blockade by intravenous naproxen infusion. Neuroscience. 2007; 148:573–583. PMID: 17651900.
Article96. Raith K, Hochhaus G. Drugs used in the treatment of opioid tolerance and physical dependence: a review. Int J Clin Pharmacol Ther. 2004; 42:191–203. PMID: 15124977.
Article97. Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005; 28:661–669. PMID: 16246435.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Pathophysiology of Primary Headaches
- Fos Protein Expression in Trigeminal Nociceptive Central Pathway of the Rat Brain by Cisternal Capsaicin Injection
- The Migraine-Stroke Connection
- The Role of Endothelial Dysfunction in the Pathophysiology and Cerebrovascular Effects of Migraine: A Narrative Review
- Pathophysiology of Vestibular Migraine