Cancer Res Treat.
2018 Oct;50(4):1121-1129. 10.4143/crt.2017.329.
HBsAg-Negative, Anti-HBc–Negative Patients Still Have a Risk of Hepatitis B Virus–Related Hepatitis after Autologous Stem Cell Transplantation for Multiple Myeloma or Malignant Lymphoma
- Affiliations
-
- 1Division of Hematology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. hemakim@yuhs.ac
- 2Division of Gastroenterology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2424785
- DOI: http://doi.org/10.4143/crt.2017.329
Abstract
- PURPOSE
Although hepatitis B surface antigen (HBsAg)-negative, hepatitis B core antibody (anti-HBc)-negative patients are not considered to be at risk for hepatitis B virus (HBV)-related hepatitis, the actual risk remains to be elucidated. This study aimed to evaluate the risk of HBV-related hepatitis in HBsAg-negative, anti-HBc-negative patients receiving autologous stem cell transplantation (ASCT) for multiple myeloma (MM) or malignant lymphoma.
MATERIALS AND METHODS
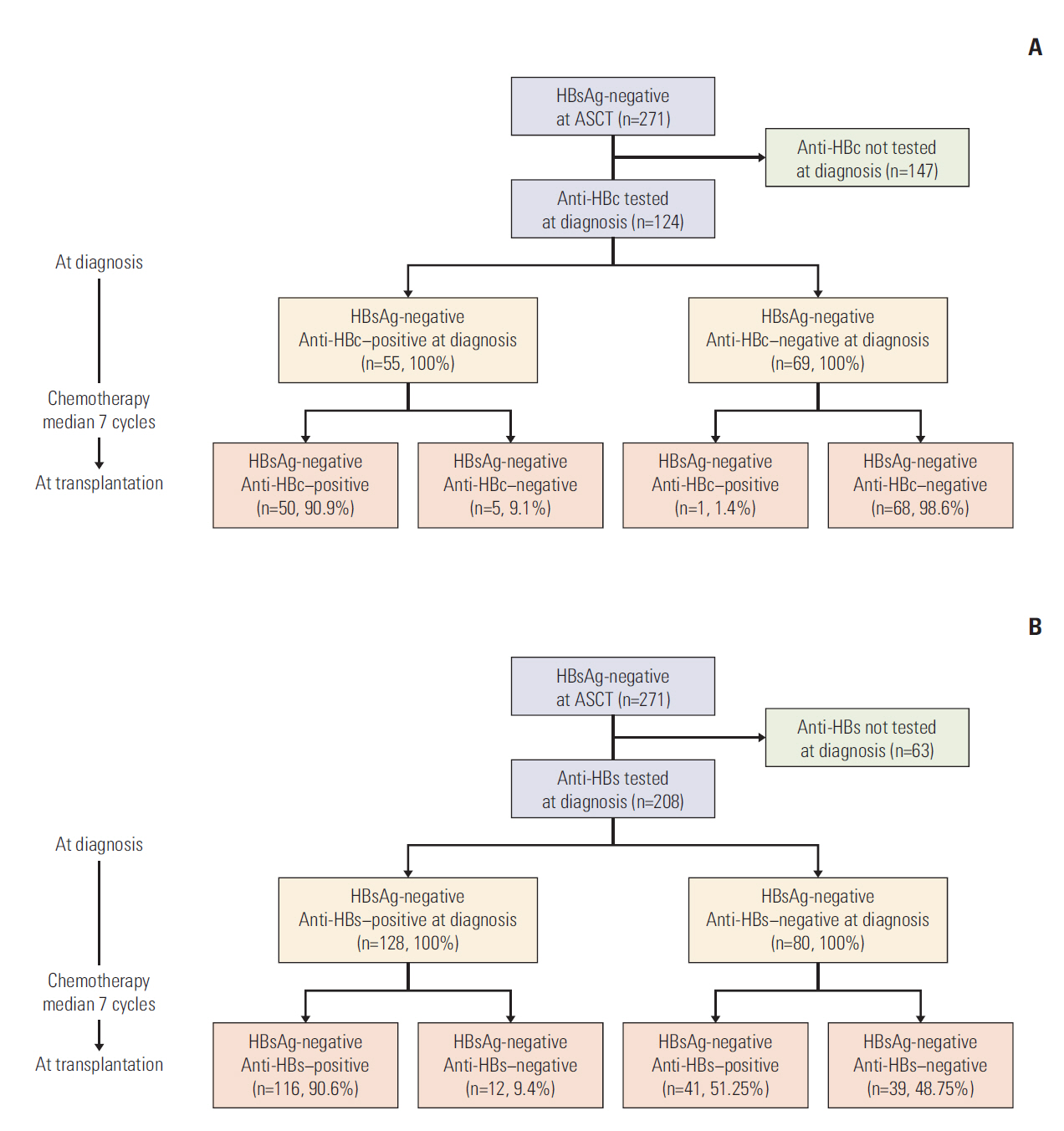
We retrospectively reviewed data from 271 HBsAg-negative patients (161 anti-HBc-negative and 110 anti-HBc-positive at the time of ASCT) who received ASCT for MM or lymphoma. The risk of HBV-related hepatitis was analyzed according to the presence of anti-HBc. HBV serology results at the time of ASCT were compared with those at the time of diagnosis of MM or lymphoma.
RESULTS
Three patients (two anti-HBc-negative MMs and one anti-HBc-positive MM) developed HBV-related hepatitis after ASCT. The rate of HBV-related hepatitis did not differ among patients with or without anti-HBc status (p=0.843). HBV-related hepatitis more frequently occurred in MM patients than in lymphoma patients (p=0.041). Overall, 9.1% of patients (16.7% with MM and 5.4% with lymphoma) who were HBsAg-negative and anti-HBc-positive at the time of diagnosis had lost anti-HBc positivity during chemotherapy prior to ASCT.
CONCLUSION
Our data suggest that HBsAg-negative, anti-HBc-negative patients at the time of ASCT for MM or lymphoma still might be at a risk for HBV-related hepatitis.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Pei SN, Chen CH. Risk and prophylaxis strategy of hepatitis B virus reactivation in patients with lymphoma undergoing chemotherapy with or without rituximab. Leuk Lymphoma. 2015; 56:1611–8.
Article2. Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014; 11:209–19.
Article3. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009; 50:661–2.
Article4. Gu HR, Shin DY, Choi HS, Moon CH, Park SC, Kang HJ. HBV reactivation in a HBsAg-negative patient with multiple myeloma treated with prednisolone maintenance therapy after autologous HSCT. Blood Res. 2015; 50:51–3.
Article5. Yoshida T, Kusumoto S, Inagaki A, Mori F, Ito A, Ri M, et al. Reactivation of hepatitis B virus in HBsAg-negative patients with multiple myeloma: two case reports. Int J Hematol. 2010; 91:844–9.
Article6. Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000; 62:299–307.
Article7. European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012; 57:167–85.8. Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012; 6:531–61.
Article9. Ceneli O, Ozkurt ZN, Acar K, Rota S, Aki SZ, Yegin ZA, et al. Hepatitis B-related events in autologous hematopoietic stem cell transplantation recipients. World J Gastroenterol. 2010; 16:1765–71.10. Guillaume T, Rubinstein DB, Symann M. Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood. 1998; 92:1471–90.
Article11. Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009; 27:605–11.
Article12. Laperche S, Guitton C, Smilovici W, Courouce AM. Blood donors infected with the hepatitis B virus but persistently lacking antibodies to the hepatitis B core antigen. Vox Sang. 2001; 80:90–4.
Article13. Ponde RA, Cardoso DD, Ferro MO. The underlying mechanisms for the 'anti-HBc alone' serological profile. Arch Virol. 2010; 155:149–58.
Article14. Lu S, Xu Y, Mu Q, Cao L, Chen J, Zhu Z, et al. The risk of hepatitis B virus reactivation and the role of antiviral prophylaxis in hepatitis B surface antigen negative/hepatitis B core anti-body positive patients with diffuse large B-cell lymphoma receiving rituximab-based chemotherapy. Leuk Lymphoma. 2015; 56:1027–32.
Article15. Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014; 59:2092–100.
Article16. Kang SY, Kim MH, Lee WI. The prevalence of "anti-HBc alone" and HBV DNA detection among anti-HBc alone in Korea. J Med Virol. 2010; 82:1508–14.
Article17. Yoo JJ, Cho EJ, Cho YY, Lee M, Lee DH, Cho Y, et al. Efficacy of antiviral prophylaxis in HBsAg-negative, anti-HBc positive patients undergoing hematopoietic stem cell transplantation. Liver Int. 2015; 35:2530–6.
Article18. Papastergiou V, Lombardi R, MacDonald D, Tsochatzis EA. Global epidemiology of hepatitis B virus (HBV) infection. Curr Hepatol Rep. 2015; 14:171–8.
Article19. Han JW, Yang H, Lee HL, Bae SH, Choi JY, Lee JW, et al. Risk factors and outcomes of hepatitis B virus reactivation in hepatitis B surface antigen negative patients with hematological malignancies. Hepatol Res. 2016; 46:657–68.
Article20. Uhm JE, Kim K, Lim TK, Park BB, Park S, Hong YS, et al. Changes in serologic markers of hepatitis B following autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007; 13:463–8.
Article21. Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014; 32:3736–43.
Article22. Liossis SN, Sfikakis PP. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol. 2008; 127:280–5.
Article23. Kim H, Shin AR, Chung HH, Kim MK, Lee JS, Shim JJ, et al. Recent trends in hepatitis B virus infection in the general Korean population. Korean J Intern Med. 2013; 28:413–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HBV reactivation in a HBsAg-negative patient with multiple myeloma treated with prednisolone maintenance therapy after autologous HSCT
- Reverse Seroconversion of Hepatitis B following Allogenic Hematopoietic Stem Cell Transplantation from a Hepatitis Immune Donor in a Multiple Myeloma Patient
- Prevalence of Hepatitis B Virus and HIV Co-infection in Korea
- A Case of Hepatitis B Virus Reactivation in a HBsAg-Negative and Anti-HBs-Positive Patient with Diffuse Large B-Cell Lymphoma after Rituximab plus CHOP Chemotherapy
- Viral Serologic Markers in Patients with Acute and Chronic Liver Diseases