Brain Tumor Res Treat.
2018 Oct;6(2):60-67. 10.14791/btrt.2018.6.e14.
Surgical Outcomes of Thalamic Tumors in Children: The Importance of Diffusion Tensor Imaging, Neuro-Navigation and Intraoperative Neurophysiological Monitoring
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. nsthomas@snu.ac.kr
- 2Division of Pediatric Neurosurgery, Seoul National University Children's Hospital, Seoul, Korea.
- 3Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea.
- 4Regional Emergency Medical Center, Seoul National University Hospital, Seoul, Korea.
- 5Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 6Division of Pediatric Radiology, Seoul National University Children's Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 7Department of Neurosurgery, The Armed Forces Capital Hospital, Seongnam, Korea.
- KMID: 2423975
- DOI: http://doi.org/10.14791/btrt.2018.6.e14
Abstract
- BACKGROUND
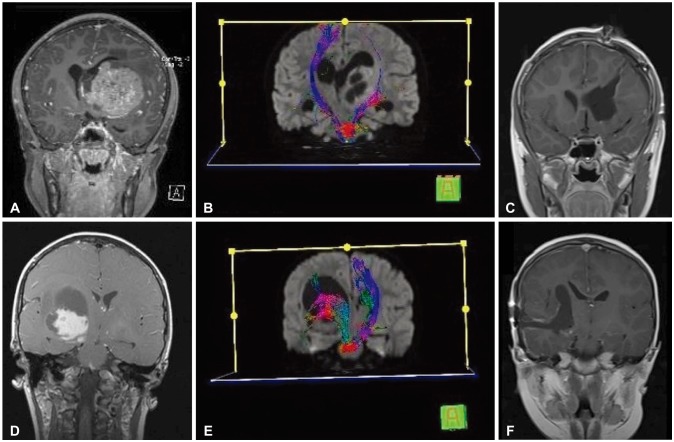
Recently, modern technology such as diffusion tensor imaging (DTI), neuro-navigation and intraoperative neurophysiological monitoring (IOM) have been actively adopted for the treatment of thalamic tumors. We evaluated surgical outcomes and efficacy of the aforementioned technologies for the treatment of pediatric thalamic tumors.
METHODS
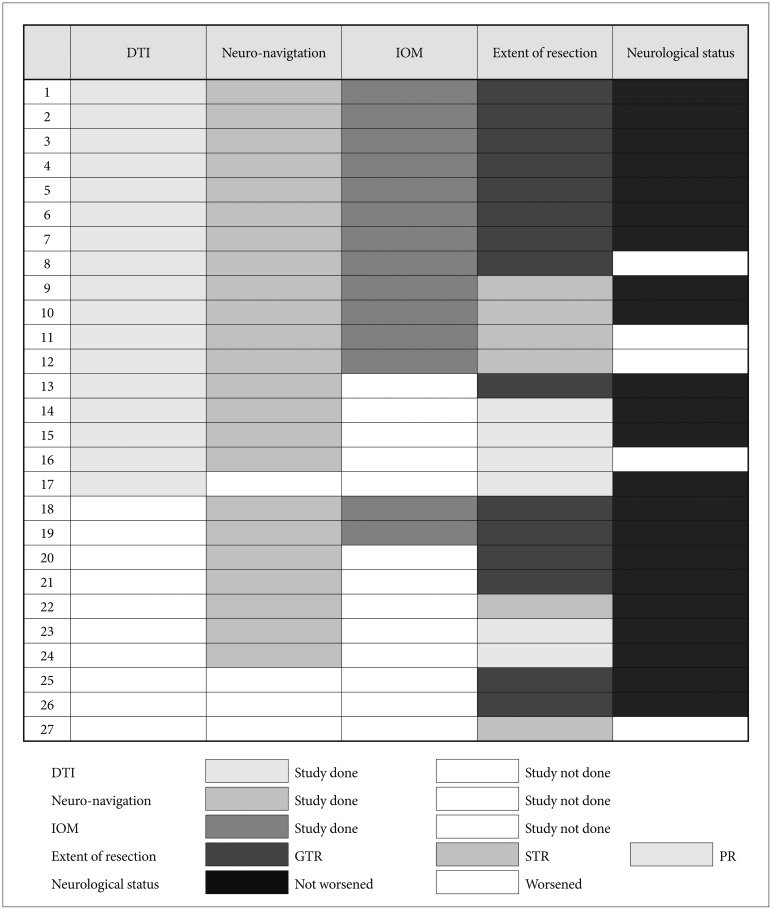
We retrospectively reviewed clinical data from 37 children with thalamic tumors between 2004 and 2017. There were 44 operations (27 tumor resections, 17 biopsies). DTI was employed in 17 cases, neuro-navigation in 23 cases and IOM in 14 cases. All diagnoses were revised according to the 2016 World Health Organization Classification of Tumors of the Central Nervous System. Progression-free survival (PFS) and overall survival (OS) rates were calculated, and relevant prognostic factors were analyzed. The median follow-up duration was 19 months.
RESULTS
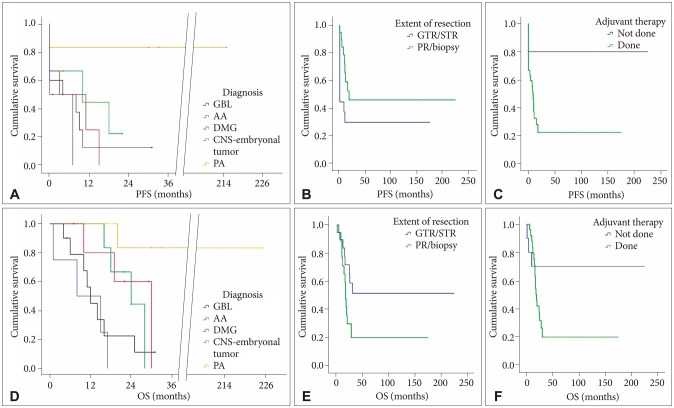
Fifteen cases were gross total resections (GTR), 6 subtotal resections (STR), and 6 partial resections (PR). Neurological status did not worsen after 22 tumor resections. There were statistically significant differences in terms of the extent of resection between the groups with DTI, neuro-navigation and IOM (n=12, GTR or STR=12) and the group without at least one of the three techniques (n=15, GTR or STR=9, p=0.020). The mean PFS was 87.2±38.0 months, and the mean OS 90.7±36.1 months. The 5-year PFS was 37%, and the 5-year OS 47%. The histological grade (p≤0.001) and adjuvant therapy (done vs. not done, p=0.016) were significantly related to longer PFS. The histological grade (p=0.002) and the extent of removal (GTR/STR vs. PR/biopsy, p=0.047) were significantly related to longer OS.
CONCLUSION
Maximal surgical resection was achieved with acceptable morbidity in children with thalamic tumors by employing DTI, neuro-navigation and IOM. Maximal tumor resection was a relevant clinical factor affecting OS; therefore, it should be considered the initial therapeutic option for pediatric thalamic tumors.
Keyword
MeSH Terms
Figure
Reference
-
1. Colosimo C, di Lella GM, Tartaglione T, Riccardi R. Neuroimaging of thalamic tumors in children. Childs Nerv Syst. 2002; 18:426–439. PMID: 12192502.
Article2. Cuccia V, Monges J. Thalamic tumors in children. Childs Nerv Syst. 1997; 13:514–520. PMID: 9403198.
Article3. Di Rocco C, Iannelli A. Bilateral thalamic tumors in children. Childs Nerv Syst. 2002; 18:440–444. PMID: 12192503.
Article4. Fernandez C, Maues de Paula A, Colin C, et al. Thalamic gliomas in children: an extensive clinical, neuroradiological and pathological study of 14 cases. Childs Nerv Syst. 2006; 22:1603–1610. PMID: 16951965.
Article5. Puget S, Crimmins DW, Garnett MR, et al. Thalamic tumors in children: a reappraisal. J Neurosurg. 2007; 106(5 Suppl):354–362. PMID: 17566201.
Article6. Jeelani O, Dirks P. Thalamic tumors. In : Richard Winn H, editor. Youmans and Winn neurological surgery. 7th ed. Elsevier;2017. Ch.208.7. Bilginer B, Narin F, Iśıkay I, Oguz KK, Söylemezoglu F, Akalan N. Thalamic tumors in children. Childs Nerv Syst. 2014; 30:1493–1498. PMID: 24752707.
Article8. Zinn PO, Colen RR, Kasper EM, Burkhardt JK. Extent of resection and radiotherapy in GBM: A 1973 to 2007 surveillance, epidemiology and end results analysis of 21,783 patients. Int J Oncol. 2013; 42:929–934. PMID: 23338774.
Article9. Steiger HJ, Götz C, Schmid-Elsaesser R, Stummer W. Thalamic astrocytomas: surgical anatomy and results of a pilot series using maximum microsurgical removal. Acta Neurochir (Wien). 2000; 142:1327–1336. PMID: 11214625.
Article10. Kis D, Máté A, Kincses ZT, Vörös E, Barzó P. The role of probabilistic tractography in the surgical treatment of thalamic gliomas. Neurosurgery. 2014; 10(Suppl 2):262–272. PMID: 24594925.
Article11. Kramm CM, Butenhoff S, Rausche U, et al. Thalamic high-grade gliomas in children: a distinct clinical subset? Neuro Oncol. 2011; 13:680–689. PMID: 21636712.
Article12. Steinbok P, Gopalakrishnan CV, Hengel AR, et al. Pediatric thalamic tumors in the MRI era: a Canadian perspective. Childs Nerv Syst. 2016; 32:269–280. PMID: 26597682.
Article13. Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003; 30(6 Suppl 19):10–14.
Article14. Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993; 26:239–244. PMID: 8387988.
Article15. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131:803–820. PMID: 27157931.
Article16. Sai Kiran NA, Thakar S, Dadlani R, et al. Surgical management of thalamic gliomas: case selection, technical considerations, and review of literature. Neurosurg Rev. 2013; 36:383–393. PMID: 23354786.
Article17. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996. PMID: 15758009.
Article18. Tarbell NJ, Friedman H, Polkinghorn WR, et al. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol. 2013; 31:2936–2941. PMID: 23857975.
Article19. Bernstein M, Hoffman HJ, Halliday WC, Hendrick EB, Humphreys RP. Thalamic tumors in children. Long-term follow-up and treatment guidelines. J Neurosurg. 1984; 61:649–656. PMID: 6088730.20. Beks JW, Bouma GJ, Journée HL, et al. Tumours of the thalamic region. A retrospective study of 27 cases. Acta Neurochir (Wien). 1987; 85:125–127. PMID: 3591474.21. Cinalli G, Aguirre DT, Mirone G, et al. Surgical treatment of thalamic tumors in children. J Neurosurg Pediatr. 2018; 21:247–257. PMID: 29271729.
Article22. Moshel YA, Elliott RE, Monoky DJ, Wisoff JH. Role of diffusion tensor imaging in resection of thalamic juvenile pilocytic astrocytoma. J Neurosurg Pediatr. 2009; 4:495–505. PMID: 19951034.
Article23. Tournier JD, Calamante F, King MD, Gadian DG, Connelly A. Limitations and requirements of diffusion tensor fiber tracking: an assessment using simulations. Magn Reson Med. 2002; 47:701–708. PMID: 11948731.
Article24. Bizzi A. Presurgical mapping of verbal language in brain tumors with functional MR imaging and MR tractography. In : Pia Sundgren M, editor. Advanced imaging techniques in brain tumors. Elsevier;2009. p. 573–596.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Principle and Experiments in Diffusion Tensor Imaging
- Commentary on “Intraoperative Monitoring for Cauda Equina Tumors: Surgical Outcomes and Neurophysiological Data Accrued Over 10 Years”
- Clinical practice guidelines for intraoperative neurophysiological monitoring: 2020 update
- Advanced Magnetic Resonance Imaging for Pediatric Brain Tumors: Current Imaging Techniques and Interpretation Algorithms
- Advanced MRI for Pediatric Brain Tumors with Emphasis on Clinical Benefits