Ann Surg Treat Res.
2018 Nov;95(5):286-296. 10.4174/astr.2018.95.5.286.
The long-term prognostic impact of sentinel lymph node biopsy in patients with primary cutaneous melanoma: a prospective study with 10-year follow-up
- Affiliations
-
- 1Department of Surgery and Department of Morphology, Surgery and Experimental Medicine, S. Anna University Hospital and University of Ferrara, Ferrara, Italy. mattia.portinari@unife.it
- 2Department of Anesthesia, McGill University Health Centre, Montreal General Hospital, Montreal, Canada.
- 3Immunotherapy and Somatic Cell Therapy Unit, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola (FC), Italy.
- 4Department of Medical Sciences, Section of Dermatology, S. Anna University Hospital and University of Ferrara, Ferrara, Italy.
- 5Unit of Nuclear Medicine, Department of Diagnostic Imaging, S. Anna University Hospital, Ferrara, Italy.
- 6Division for Endocrine and Minimally Invasive Surgery, Department of Human Pathology in Adulthood and Childhood “G. Barresiâ€, University Hospital “G. Martinoâ€, University of Messina, Messina, Italy.
- KMID: 2422938
- DOI: http://doi.org/10.4174/astr.2018.95.5.286
Abstract
- PURPOSE
Sentinel lymph node (SLN) biopsy (SLNB) is widely accepted for staging of melanoma patients. It has been shown that clinico-pathological features such as Breslow thickness, ulceration, age, and sex are better predictors of relapse and survival than SLN status alone. The aims of this study were to evaluate the long-term (10-year) prognostic impact of SLNB and to determine predictive factors associated with SLN metastasis, relapse, and melanoma specific mortality (MSM).
METHODS
This was a prospective observational study on 289 consecutive patients with primary cutaneous melanoma who underwent SLNB from January 2000 to December 2007, and followed until January 2014, at an Italian academic hospital.
RESULTS
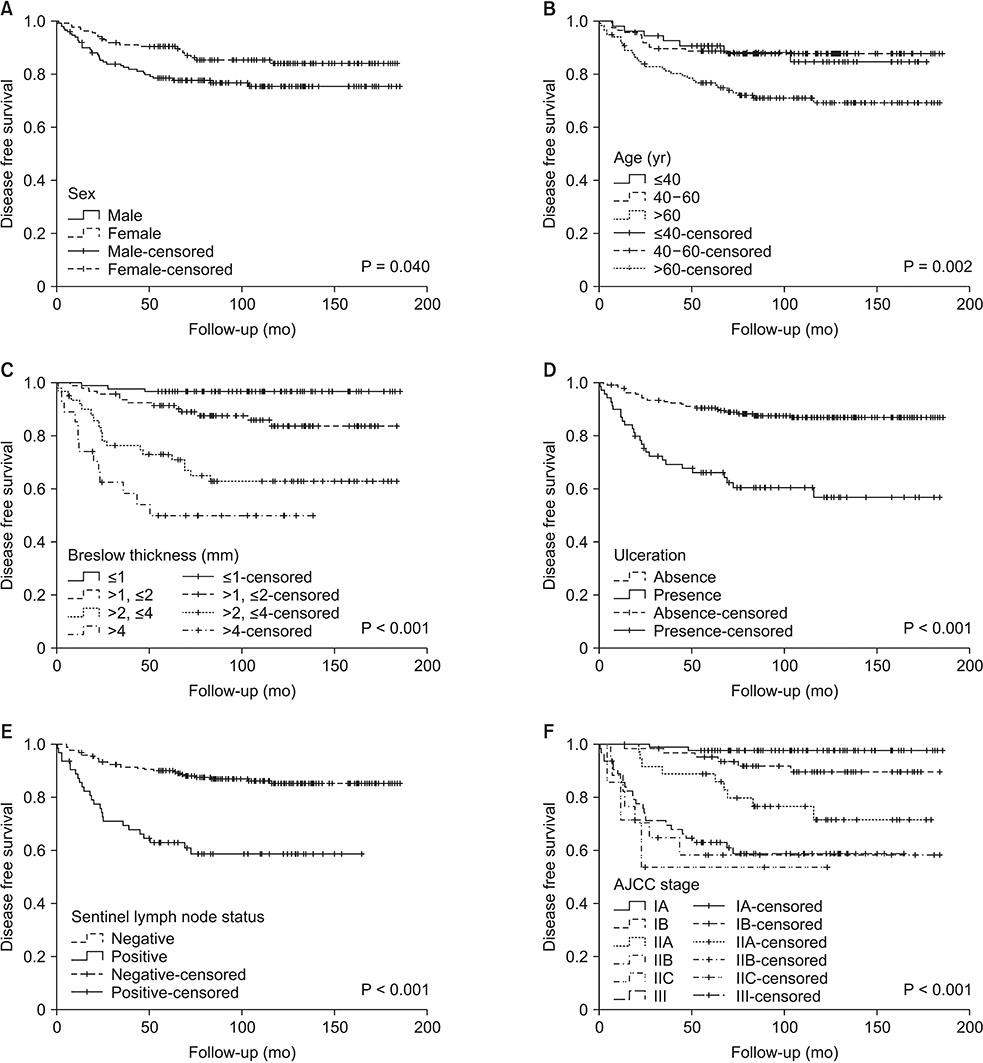
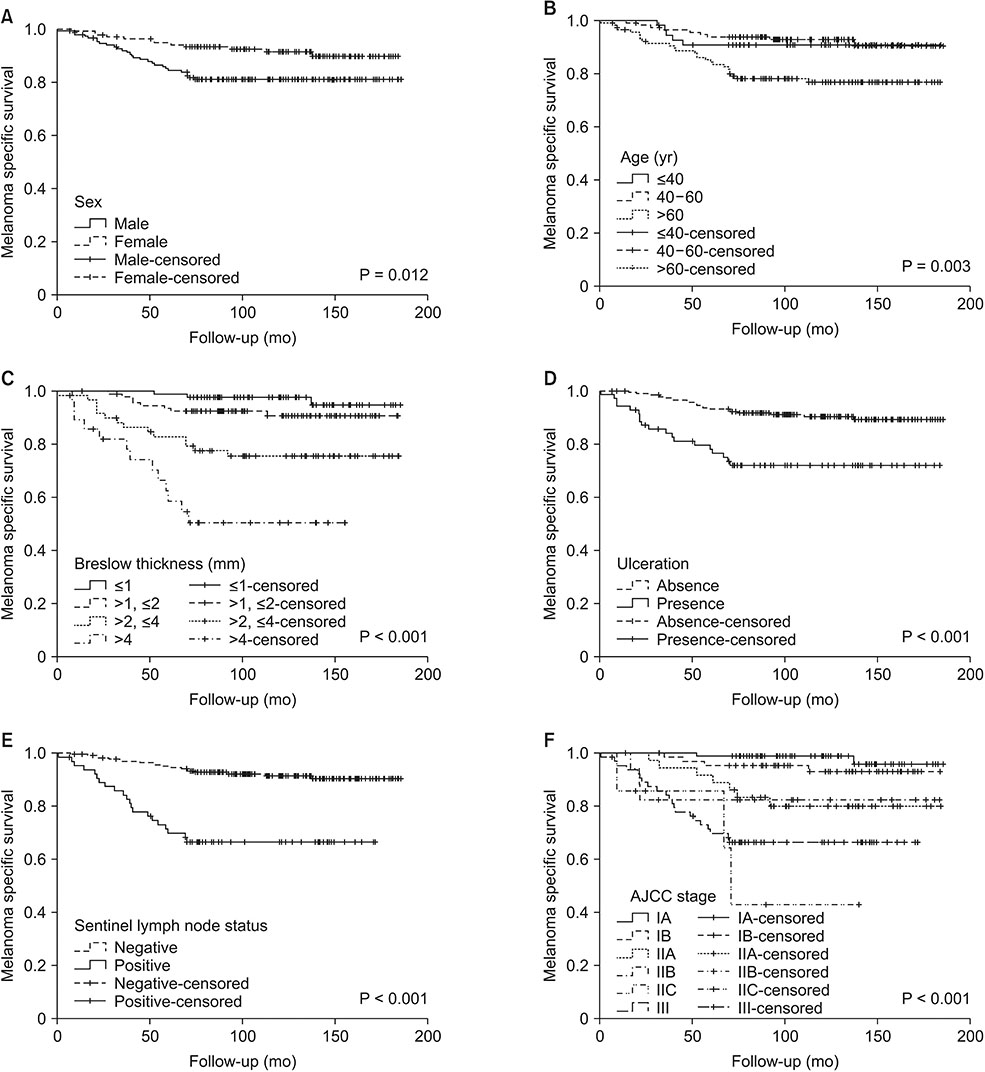
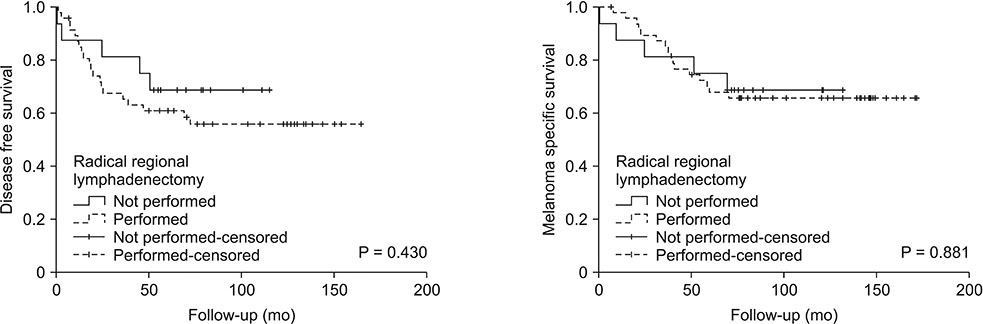
SLN was positive in 64 patients (22.1%). The median follow-up was 116 months (79-147 months). Ten-year disease-free survival and melanoma specific survival were poor in patients with positive SLN (58.7% and 66.4%, respectively). Only the increasing Breslow thickness resulted independently associated to an increased risk of SLN metastasis. Cox regression analysis showed that a Breslow thickness >2 mm was an independent predictor of relapse, and male sex and Breslow thickness >2 mm was a predictor of MSM. At 10 years, SLN metastasis was not significantly associated to either relapse or MSM.
CONCLUSION
After the fifth year of follow-up, SLN metastasis is not an independent predictive factor of relapse or mortality which are mainly influenced by the characteristics of the primary tumor and of the patient. Patients with a Breslow thickness >2 mm regardless of the SLN status should be considered at high risk for 10-year relapse and mortality.
MeSH Terms
Figure
Reference
-
1. Mozzillo N, Caracò C, Chiofalo MG, Celentano E, Lastoria S, Botti G, et al. Sentinel lymph node biopsy in patients with cutaneous melanoma: outcome after 3-year follow-up. Eur J Surg Oncol. 2004; 30:440–443.
Article2. Scoggins CR, Bowen AL, Martin RC 2nd, Edwards MJ, Reintgen DS, Ross MI, et al. Prognostic information from sentinel lymph node biopsy in patients with thick melanoma. Arch Surg. 2010; 145:622–627.
Article3. National Comprehensive Cancer Network. Melanoma (version 1.2018) [Internet]. Fort Wathington (PA): National Comprehensive Cancer Network;c2017. cited 2017 Apr 12. Available from: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf.4. Topar G, Eisendle K, Zelger B, Fritsch P. Sentinel lymph node status in melanoma: a valuable prognostic factor? Br J Dermatol. 2006; 154:1080–1087.
Article5. Mitra A, Conway C, Walker C, Cook M, Powell B, Lobo S, et al. Melanoma sentinel node biopsy and prediction models for relapse and overall survival. Br J Cancer. 2010; 103:1229–1236.
Article6. Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014; 370:599–609.
Article7. Sladden M, Zagarella S, Popescu C, Bigby M. No survival benefit for patients with melanoma undergoing sentinel lymph node biopsy: critical appraisal of the Multicenter Selective Lymphadenectomy Trial-I final report. Br J Dermatol. 2015; 172:566–571.
Article8. Kyrgidis A, Tzellos T, Mocellin S, Apalla Z, Lallas A, Pilati P, et al. Sentinel lymph node biopsy followed by lymph node dissection for localised primary cutaneous melanoma. Cochrane Database Syst Rev. 2015; (5):CD010307.
Article9. Rossi CR, De Salvo GL, Trifiro G, Mocellin S, Landi G, Macripò G, et al. The impact of lymphoscintigraphy technique on the outcome of sentinel node biopsy in 1,313 patients with cutaneous melanoma: an Italian Multicentric Study (SOLISM-IMI). J Nucl Med. 2006; 47:234–241.10. Testori A, De Salvo GL, Montesco MC, Trifiro G, Mocellin S, Landi G, et al. Clinical considerations on sentinel node biopsy in melanoma from an Italian multicentric study on 1,313 patients (SOLISM-IMI). Ann Surg Oncol. 2009; 16:2018–2027.
Article11. Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996; 14:7–17.
Article12. Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27:6199–6206.
Article13. Gershenwald JE, Soong SJ, Balch CM. American Joint Committee on Cancer (AJCC) Melanoma Staging Committee. 2010 TNM staging system for cutaneous melanoma...and beyond. Ann Surg Oncol. 2010; 17:1475–1477.
Article14. de Vries M, Speijers MJ, Bastiaannet E, Plukker JT, Brouwers AH, van Ginkel RJ, et al. Long-term follow-up reveals that ulceration and sentinel lymph node status are the strongest predictors for survival in patients with primary cutaneous melanoma. Eur J Surg Oncol. 2011; 37:681–687.
Article15. Jones EL, Jones TS, Pearlman NW, Gao D, Stovall R, Gajdos C, et al. Long-term follow-up and survival of patients following a recurrence of melanoma after a negative sentinel lymph node biopsy result. JAMA Surg. 2013; 148:456–461.
Article16. Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017; 376:2211–2222.17. White RL Jr, Ayers GD, Stell VH, Ding S, Gershenwald JE, Salo JC, et al. Factors predictive of the status of sentinel lymph nodes in melanoma patients from a large multicenter database. Ann Surg Oncol. 2011; 18:3593–3600.
Article18. Bartlett EK, Karakousis GC. Current staging and prognostic factors in melanoma. Surg Oncol Clin N Am. 2015; 24:215–227.
Article19. Chao C, Martin RC 2nd, Ross MI, Reintgen DS, Edwards MJ, Noyes RD, et al. Correlation between prognostic factors and increasing age in melanoma. Ann Surg Oncol. 2004; 11:259–264.
Article20. Carlson GW. Age and the incidence of sentinel lymph node metastases in melanoma. Ann Surg Oncol. 2004; 11:236–237.
Article21. Oliveria SA, Christos PJ, Halpern AC, Fine JA, Barnhill RL, Berwick M. Evaluation of factors associated with skin self-examination. Cancer Epidemiol Biomarkers Prev. 1999; 8:971–978.22. Testori A, Stanganelli I, Della Grazia L, Mahadavan L. Diagnosis of melanoma in the elderly and surgical implications. Surg Oncol. 2004; 13:211–221.
Article23. Christos PJ, Oliveria SA, Berwick M, Guerry D4th, Elder DE, Synnestvedt M, et al. Signs and symptoms of melanoma in older populations. J Clin Epidemiol. 2000; 53:1044–1053.
Article24. Valsecchi ME, Silbermins D, de Rosa N, Wong SL, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: a meta-analysis. J Clin Oncol. 2011; 29:1479–1487.
Article25. van Akkooi AC, de Wilt JH, Verhoef C, Graveland WJ, van Geel AN, Kliffen M, et al. High positive sentinel node identification rate by EORTC melanoma group protocol. Prognostic indicators of metastatic patterns after sentinel node biopsy in melanoma. Eur J Cancer. 2006; 42:372–380.26. Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001; 19:3622–3634.
Article27. Balch CM, Sober AJ, Soong SJ, Gershenwald JE. AJCC Melanoma Staging Committee. The new melanoma staging system. Semin Cutan Med Surg. 2003; 22:42–54.
Article28. Murali R, Desilva C, Thompson JF, Scolyer RA. Factors predicting recurrence and survival in sentinel lymph node-positive melanoma patients. Ann Surg. 2011; 253:1155–1164.
Article29. Zogakis TG, Essner R, Wang HJ, Foshag LJ, Morton DL. Natural history of melanoma in 773 patients with tumor-negative sentinel lymph nodes. Ann Surg Oncol. 2007; 14:1604–1611.
Article30. Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng CH, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999; 17:976–983.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary cutaneous malignant melanoma of the breast
- Management of Malignant Melanoma Using Sentinel Lymph Node Biopsy: A Case Report
- Treatment of Malignant Melanoma Using Sentinel Lymph Node Dissection
- Minimally Invasive Surgery Based on Sentinel Node Biopsy for Gastrointestinal Cancer
- Cutaneous melanoma