J Pathol Transl Med.
2018 Sep;52(5):307-313. 10.4132/jptm.2018.08.03.
Interleukin-31, Interleukin-31RA, and OSMR Expression Levels in Post-burn Hypertrophic Scars
- Affiliations
-
- 1Department of Pathology, Hallym University College of Medicine, Chuncheon, Korea.
- 2Department of Pathology, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea.
- 3Department of Anesthesiology and Pain Medicine, Hallym University Hangang Sacred Heart Hospital, Seoul, Korea.
- 4Department of Pathology, Hallym University Hangang Sacred Heart Hospital, Research Institute for Complementary and Alternative Medicine, Seoul, Korea. yhchoi@hallym.or.kr
- KMID: 2422097
- DOI: http://doi.org/10.4132/jptm.2018.08.03
Abstract
- BACKGROUND
Although several studies have shown the role of interleukin-31 (IL-31) and its receptors in inducing pruritus in certain skin disorders, knowledge of its role in post-burn hypertrophic scars is insufficient. Therefore, the histopathological expression levels of IL-31, IL-31 receptor alpha (IL-31RA), and oncostatin M receptor (OSMR) in post-burn hypertrophic scar tissues were investigated and compared with normal tissue expression levels.
METHODS
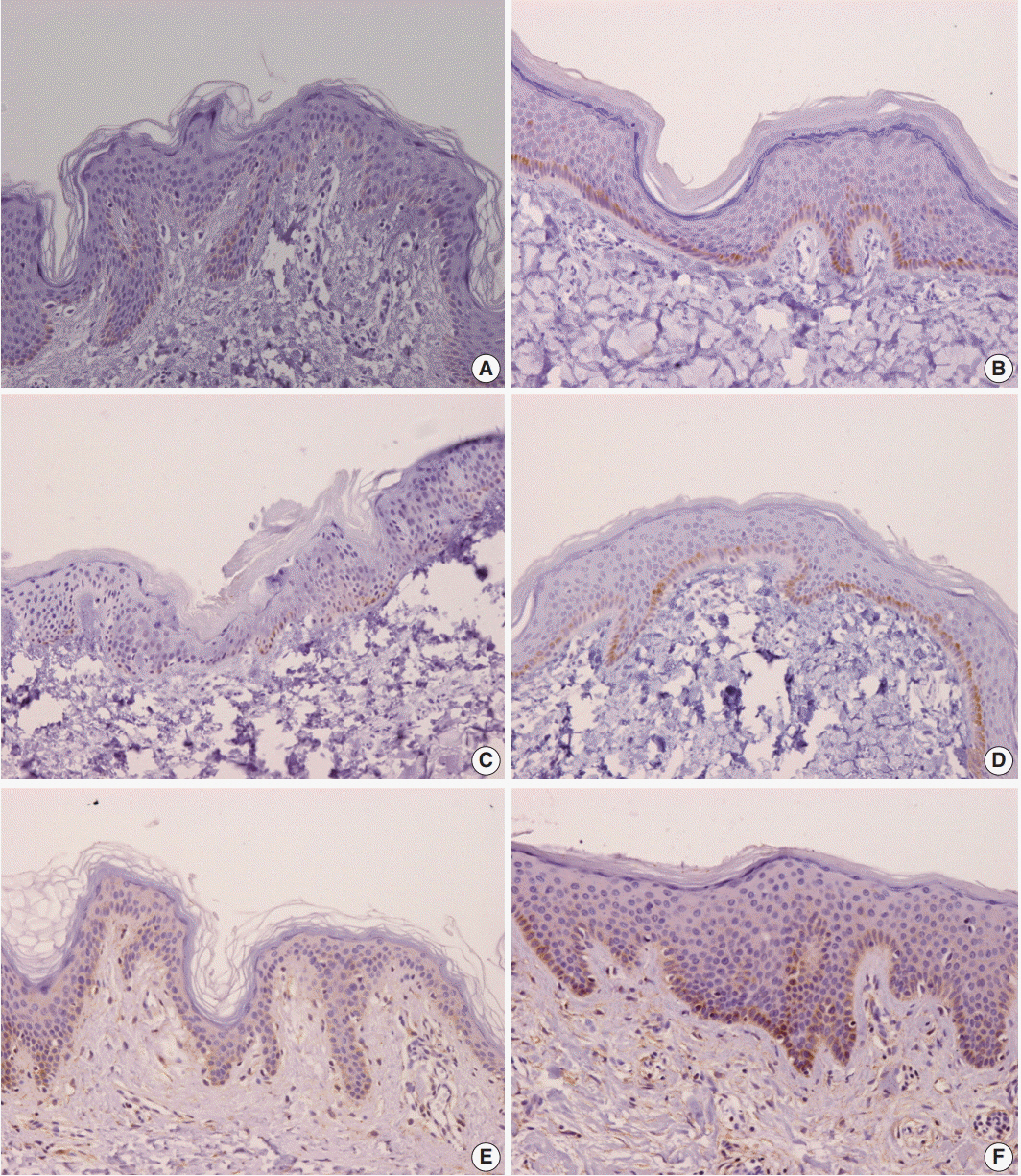
Samples of hypertrophic scar tissue were obtained from 20 burn patients through punch biopsy. Normal samples were obtained from areas adjacent to the burn injury site of the same patients. Samples were placed in 10% neutral buffered formalin, embedded in paraplast, and processed into serial 5-μm sections. Immunohistochemistry results were semi-quantitatively evaluated for IL-31, IL-31RA, and OSMR. By hematoxylin and eosin staining, epidermal and dermal thickness were assessed with a microscope and digital camera. Intensities were rated on a scale of 1 to 4.
RESULTS
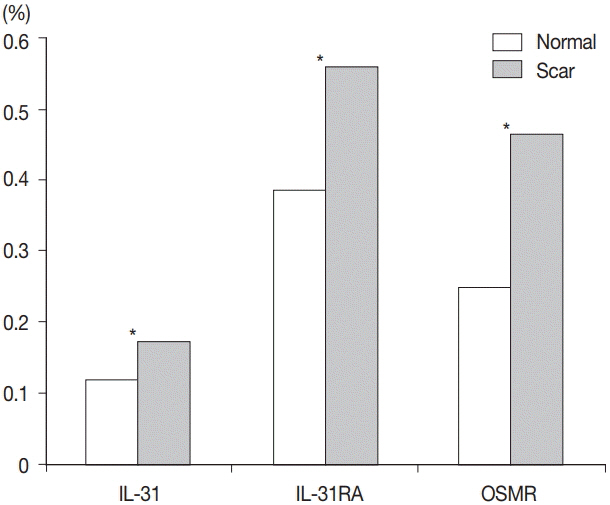
Percentages for IL-31, IL-31RA, and OSMR in the epidermal basal layer cell cytoplasm were significantly greater in the burn scar tissue compared to normal skin, as well as the dermal and epidermal thickness (p < .05). There was a significant difference in IL-31 epidermal basal layer intensity in burn scar tissue compared to normal skin (p < .05). Besides the OSMR basal layer intensity, IL-31 and IL-31RA intensities between the burn scar and normal tissues were not significant. However, correlations were significant, indicating that the greater the infiltration percentage, the higher the intensity (p < .05).
CONCLUSIONS
IL-31, IL-31RA, and OSMR expression levels are increased in hypertrophic scars compared with normal tissue.
Keyword
MeSH Terms
Figure
Reference
-
1. Amini-Nik S, Yousuf Y, Jeschke MG. Scar management in burn injuries using drug delivery and molecular signaling: current treatments and future directions. Adv Drug Deliv Rev. 2018; 123:135–54.
Article2. Bombaro KM, Engrav LH, Carrougher GJ, et al. What is the prevalence of hypertrophic scarring following burns? Burns. 2003; 29:299–302.
Article3. Szulgit G, Rudolph R, Wandel A, Tenenhaus M, Panos R, Gardner H. Alterations in fibroblast α1β1 integrin collagen receptor expression in keloids and hypertrophic scars. J Invest Dermatol. 2002; 118:409–15.
Article4. Knapp TR, Daniels RJ, Kaplan EN. Pathologic scar formation: morphologic and biochemical correlates. Am J Pathol. 1977; 86:47–70.5. Swann DA, Garg HG, Jung W, Hermann H. Studies on human scar tissue proteoglycans. J Invest Dermatol. 1985; 84:527–31.
Article6. Shetlar MR, Dobrkovsky M, Linares H, Villarante R, Shetlar CL, Larson DL. The hypertrophic scar. Glycoprotein and collagen components of burn scars. Proc Soc Exp Biol Med. 1971; 138:298–300.
Article7. Babu M, Diegelmann R, Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989; 9:1642–50.
Article8. Murray JC. Keloids and hypertrophic scars. Clin Dermatol. 1994; 12:27–37.
Article9. McCauley RL, Chopra V, Li YY, Herndon DN, Robson MC. Altered cytokine production in black patients with keloids. J Clin Immunol. 1992; 12:300–8.
Article10. Ghahary A, Shen YJ, Scott PG, Gong Y, Tredget EE. Enhanced expression of mRNA for transforming growth factor-beta, type I and type III procollagen in human post-burn hypertrophic scar tissues. J Lab Clin Med. 1993; 122:465–73.11. Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on Abnormal Scarring. Adv Wound Care (New Rochelle). 2015; 4:119–36.
Article12. Choi YH, Kim KM, Kim HO, Jang YC, Kwak IS. Clinical and histological correlation in post-burn hypertrophic scar for pain and itching sensation. Ann Dermatol. 2013; 25:428–33.
Article13. Scott JR, Muangman P, Gibran NS. Making sense of hypertrophic scar: a role for nerves. Wound Repair Regen. 2007; 15 Suppl 1:S27–31.
Article14. Parnell LK, Nedelec B, Rachelska G, LaSalle L. Assessment of pruritus characteristics and impact on burn survivors. J Burn Care Res. 2012; 33:407–18.
Article15. Profyris C, Tziotzios C, Do Vale I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J Am Acad Dermatol. 2012; 66:1–10.16. Van Loey NE, Bremer M, Faber AW, Middelkoop E, Nieuwenhuis MK. Itching following burns: epidemiology and predictors. Br J Dermatol. 2008; 158:95–100.
Article17. Bilsborough J, Leung DY, Maurer M, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006; 117:418–25.
Article18. Cornelissen C, Lüscher-Firzlaff J, Baron JM, Lüscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol. 2012; 91:552–66.
Article19. Nobbe S, Dziunycz P, Mühleisen B, et al. IL-31 expression by inflammatory cells is preferentially elevated in atopic dermatitis. Acta Derm Venereol. 2012; 92:24–8.20. Sonkoly E, Muller A, Lauerma AI, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006; 117:411–7.
Article21. Hawro T, Saluja R, Weller K, Altrichter S, Metz M, Maurer M. Interleukin-31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy. 2014; 69:113–7.
Article22. Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004; 5:752–60.
Article23. Le Saux S, Rousseau F, Barbier F, et al. Molecular dissection of human interleukin-31-mediated signal transduction through site-directed mutagenesis. J Biol Chem. 2010; 285:3470–7.
Article24. Kwak IS, Choi YH, Jang YC, Lee YK. Immunohistochemical analysis of neuropeptides (protein gene product 9.5, substance P and calcitonin gene-related peptide) in hypertrophic burn scar with pain and itching. Burns. 2014; 40:1661–7.
Article25. Varney VA, Hamid QA, Gaga M, et al. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J Clin Invest. 1993; 92:644–51.
Article26. Kasraie S, Niebuhr M, Baumert K, Werfel T. Functional effects of interleukin 31 in human primary keratinocytes. Allergy. 2011; 66:845–52.
Article27. Nattkemper LA, Martinez-Escala ME, Gelman AB, et al. Cutaneous T-cell lymphoma and pruritus: the expression of IL-31 and its receptors in the skin. Acta Derm Venereol. 2016; 96:894–8.
Article28. Kasutani K, Fujii E, Ohyama S, et al. Anti-IL-31 receptor antibody is shown to be a potential therapeutic option for treating itch and dermatitis in mice. Br J Pharmacol. 2014; 171:5049–58.
Article29. Bando T, Morikawa Y, Komori T, Senba E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. 2006; 142:1263–71.30. Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Bíró T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006; 126:1705–18.
Article31. Van Cromphaut I, Fumal I, Jacquemin D, Fissette J, Piérard GE. Skin barrier repair after contact burns: electrometric evaluation using the passive sustainable hydration test. J Environ Med. 1999; 1:47–50.32. Vickery BP. Skin barrier function in atopic dermatitis. Curr Opin Pediatr. 2007; 19:89–93.
Article33. Singh B, Jegga AG, Shanmukhappa KS, et al. IL-31-driven skin remodeling involves epidermal cell proliferation and thickening that lead to impaired skin-barrier function. PLoS One. 2016; 11:e0161877.
Article34. Niyonsaba F, Ushio H, Hara M, et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. 2010; 184:3526–34.35. Poindexter BJ, Bhat S, Buja LM, Bick RJ, Milner SM. Localization of antimicrobial peptides in normal and burned skin. Burns. 2006; 32:402–7.
Article36. Niyonsaba F, Nagaoka I, Ogawa H. Human defensins and cathelicidins in the skin: beyond direct antimicrobial properties. Crit Rev Immunol. 2006; 26:545–76.
Article37. Frohm M, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997; 272:15258–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Core extirpation of post-burn hypertrophic scar of the auricle
- The role of interleukin-6 and interleukin-10 in human pulpal inflammation
- Levels od serum soluble interleukin-2 receptor in patients with burn
- Effects of B-16 Melanoma Cells and Mycoplasma pneumoniae on the Induction of IL-1 beta, IL-2, IL-6, IL-10, IL-12, and TNF - alpha from Mouse Astrocytes
- The soluble interleukin 2 receptor levels in Kawasaki disease