Allergy Asthma Immunol Res.
2017 Nov;9(6):526-533. 10.4168/aair.2017.9.6.526.
Effects of Immunoglobulin Replacement on Asthma Exacerbation in Adult Asthmatics with IgG Subclass Deficiency
- Affiliations
-
- 1Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 2Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 3Department of Allergy and Clinical Immunology, Dong-A University College of Medicine, Busan, Korea.
- 4Department of Allergy and Clinical Immunology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2421663
- DOI: http://doi.org/10.4168/aair.2017.9.6.526
Abstract
- PURPOSE
Recurrent respiratory tract infection is a common manifestation of primary immunodeficiency disease, and respiratory viruses or bacteria are important triggers of asthma exacerbations. Asthma often coexists with humoral immunodeficiency in adults, and some asthmatics with immunoglobulin (Ig) G subclass deficiency (IgGSCD) suffer from recurrent exacerbations. Although some studies suggest a benefit from Ig replacement, others have failed to support its use. This study aimed to assess the effect of Ig replacement on asthma exacerbation caused by respiratory infection as well as the asthma control status of adult asthmatics with IgGSCD.
METHODS
This is a multi-center, open-label study of adult asthmatics with IgGSCD. All patients received monthly intravenous immunoglobulin (IVIG) for 6 months and were evaluated regarding asthma exacerbation related to infection, asthma control status, quality of life, and lung function before and after IVIG infusion.
RESULTS
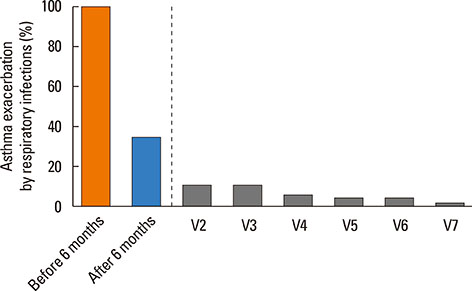
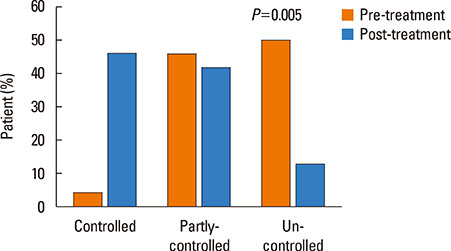
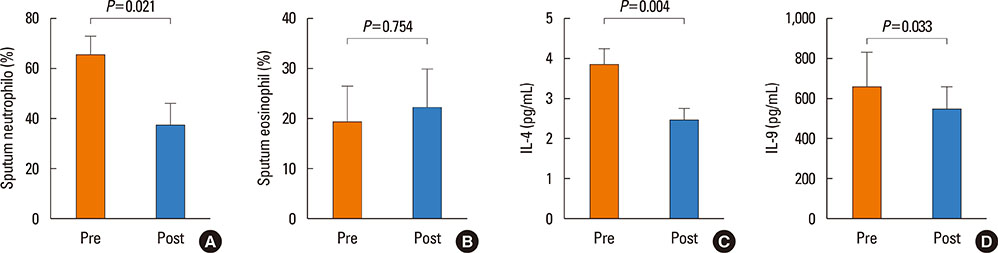
A total of 30 patients were enrolled, and 24 completed the study. Most of the patients had a moderate degree of asthma severity with partly (52%) or uncontrolled (41%) status at baseline. IVIG significantly reduced the proportion of patients with asthma exacerbations, lowered the number of respiratory infections, and improved asthma control status, compared to the baseline values (P < 0.001). The mean asthma-specific quality of life and asthma control test scores were improved significantly (P=0.009 and P=0.053, respectively); however, there were no significant changes in lung function.
CONCLUSIONS
IVIG reduced the frequency of asthma exacerbations and improved asthma control status in adult asthmatics with IgGSCD, suggesting that IVIG could be an effective treatment option in this population.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Sublingual Immunotherapy for Japanese Cedar Pollinosis Attenuates Asthma Exacerbation
Sayaka Kikkawa, Kazuyuki Nakagome, Takehito Kobayashi, Tomoyuki Soma, Atsushi Kamijo, Makoto Nagata
Allergy Asthma Immunol Res. 2019;11(3):438-440. doi: 10.4168/aair.2019.11.3.438.Familial IgG3 subclass deficiency: A report of two cases
Ji-Ho Lee, Seung-Hyun Kim, Chang-Gyu Jung, Youngwoo Choi, Hae-Sim Park
Allergy Asthma Respir Dis. 2018;6(3):184-187. doi: 10.4168/aard.2018.6.3.184.
Reference
-
1. Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009; 39:193–202.2. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14.3. Busse WW, Lemanske RF Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010; 376:826–834.4. Kurai D, Saraya T, Ishii H, Takizawa H. Virus-induced exacerbations in asthma and COPD. Front Microbiol. 2013; 4:293.5. Azar AE, Ballas ZK. Evaluation of the adult with suspected immunodeficiency. Am J Med. 2007; 120:764–768.6. Kim JH, Park HJ, Choi GS, Kim JE, Ye YM, Nahm DH, et al. Immunoglobulin G subclass deficiency is the major phenotype of primary immunodeficiency in a Korean adult cohort. J Korean Med Sci. 2010; 25:824–828.7. Abdou NI, Greenwell CA, Mehta R, Narra M, Hester JD, Halsey JF. Efficacy of intravenous gammaglobulin for immunoglobulin G subclass and/or antibody deficiency in adults. Int Arch Allergy Immunol. 2009; 149:267–274.8. Kim JH, Park S, Hwang YI, Jang SH, Jung KS, Sim YS, et al. Immunoglobulin g subclass deficiencies in adult patients with chronic airway diseases. J Korean Med Sci. 2016; 31:1560–1565.9. Agondi RC, Barros MT, Rizzo LV, Kalil J, Giavina-Bianchi P. Allergic asthma in patients with common variable immunodeficiency. Allergy. 2010; 65:510–515.10. Touw CM, van de Ven AA, de Jong PA, Terheggen-Lagro S, Beek E, Sanders EA, et al. Detection of pulmonary complications in common variable immunodeficiency. Pediatr Allergy Immunol. 2010; 21:793–805.11. Ochs HD, Hagin D. Primary immunodeficiency disorders: general classification, new molecular insights, and practical approach to diagnosis and treatment. Ann Allergy Asthma Immunol. 2014; 112:489–495.12. Abrahamian F, Agrawal S, Gupta S. Immunological and clinical profile of adult patients with selective immunoglobulin subclass deficiency: response to intravenous immunoglobulin therapy. Clin Exp Immunol. 2010; 159:344–350.13. Jakobsson T, Croner S, Kjellman NI, Pettersson A, Vassella C, Björkstén B. Slight steroid-sparing effect of intravenous immunoglobulin in children and adolescents with moderately severe bronchial asthma. Allergy. 1994; 49:413–420.14. Haque S, Boyce N, Thien FC, O’Hehir RE, Douglass J. Role of intravenous immunoglobulin in severe steroid-dependent asthma. Intern Med J. 2003; 33:341–344.15. Kishiyama JL, Valacer D, Cunningham-Rundles C, Sperber K, Richmond GW, Abramson S, et al. A multicenter, randomized, double-blind, placebo-controlled trial of high-dose intravenous immunoglobulin for oral corticosteroid-dependent asthma. Clin Immunol. 1999; 91:126–133.16. Salmun LM, Barlan I, Wolf HM, Eibl M, Twarog FJ, Geha RS, et al. Effect of intravenous immunoglobulin on steroid consumption in patients with severe asthma: a double-blind, placebo-controlled, randomized trial. J Allergy Clin Immunol. 1999; 103:810–815.17. Bernatowska-Matuszkiewicz E, Pac M, Skopcynska H, Pum M, Eibl MM. Clinical efficacy of intravenous immunoglobulin in patients with severe inflammatory chest disease and IgG3 subclass deficiency. Clin Exp Immunol. 1991; 85:193–197.18. Kupczyk M, ten Brinke A, Sterk PJ, Bel EH, Papi A, Chanez P, et al. Frequent exacerbators--a distinct phenotype of severe asthma. Clin Exp Allergy. 2014; 44:212–221.19. Koga T, Oshita Y, Kamimura T, Koga H, Aizawa H. Characterisation of patients with frequent exacerbation of asthma. Respir Med. 2006; 100:273–278.20. ten Brinke A, Sterk PJ, Masclee AA, Spinhoven P, Schmidt JT, Zwinderman AH, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005; 26:812–818.21. Kim HJ, Lee J, Kim JH, Park SY, Kwon HS, Kim TB, et al. Factors affecting recovery time of pulmonary function in hospitalized patients with acute asthma exacerbations. Allergy Asthma Immunol Res. 2016; 8:499–504.22. Chen Y, Stirling RG, Paul E, Hore-Lacy F, Thompson BR, Douglass JA. Longitudinal decline in lung function in patients with primary immunoglobulin deficiencies. J Allergy Clin Immunol. 2011; 127:1414–1417.23. Goldstein MF, Hilditch GJ, Dvorin DJ, Belecanech GA. Immunoglobulin replacement for selective IgM immunodeficiency, bronchiectasis, and asthma. Ann Allergy Asthma Immunol. 2016; 116:172–173.24. Verma N, Grimbacher B, Hurst JR. Lung disease in primary antibody deficiency. Lancet Respir Med. 2015; 3:651–660.25. Landwehr LP, Jeppson JD, Katlan MG, Esterl B, McCormick D, Hamilos DL, et al. Benefits of high-dose i.v. immunoglobulin in patients with severe steroid-dependent asthma. Chest. 1998; 114:1349–1356.26. Niggemann B, Leupold W, Schuster A, Schuster R, v Berg A, Grübl A, et al. Prospective, double-blind, placebo-controlled, multicentre study on the effect of high-dose, intravenous immunoglobulin in children and adolescents with severe bronchial asthma. Clin Exp Allergy. 1998; 28:205–210.27. Yong PL, Boyle J, Ballow M, Boyle M, Berger M, Bleesing J, et al. Use of intravenous immunoglobulin and adjunctive therapies in the treatment of primary immunodeficiencies: a working group report of and study by the Primary Immunodeficiency Committee of the American Academy of Allergy Asthma and Immunology. Clin Immunol. 2010; 135:255–263.28. Ballow M. The IgG molecule as a biological immune response modifier: mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J Allergy Clin Immunol. 2011; 127:315–323.29. Yamamoto M, Kobayashi K, Ishikawa Y, Nakata K, Funada Y, Kotani Y, et al. The inhibitory effects of intravenous administration of rabbit immunoglobulin G on airway inflammation are dependent upon Fcγ receptor IIb on CD11c(+) dendritic cells in a murine model. Clin Exp Immunol. 2010; 162:315–324.30. Massoud AH, Guay J, Shalaby KH, Bjur E, Ablona A, Chan D, et al. Intravenous immunoglobulin attenuates airway inflammation through induction of forkhead box protein 3-positive regulatory T cells. J Allergy Clin Immunol. 2012; 129:1656–1665.e3.31. Massoud AH, Yona M, Xue D, Chouiali F, Alturaihi H, Ablona A, et al. Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J Allergy Clin Immunol. 2014; 133:853–863.e5.32. Gern JE. How rhinovirus infections cause exacerbations of asthma. Clin Exp Allergy. 2015; 45:32–42.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association between primary immunodeficiency and asthma exacerbation in adult asthmatics
- Serum IgG and IgG subclass in bronchial asthma
- Serum IgG and IgG subclass in aspirin-sensitive asthma
- A case of intravenous immunoglobulin therapy in severe aspirin - sensitive asthma patient combined with IgG1 and IgG3 subclass deficiency
- IgG and IgG subclass in asthma