Asthma Biomarkers: Do They Bring Precision Medicine Closer to the Clinic?
- Affiliations
-
- 1Faculty of Medicine, Department of Allergy and Clinical Immunology, Transylvania University of Brasov, Brasov, Romania. ibrumaru@unitbv.ro
- KMID: 2421656
- DOI: http://doi.org/10.4168/aair.2017.9.6.466
Abstract
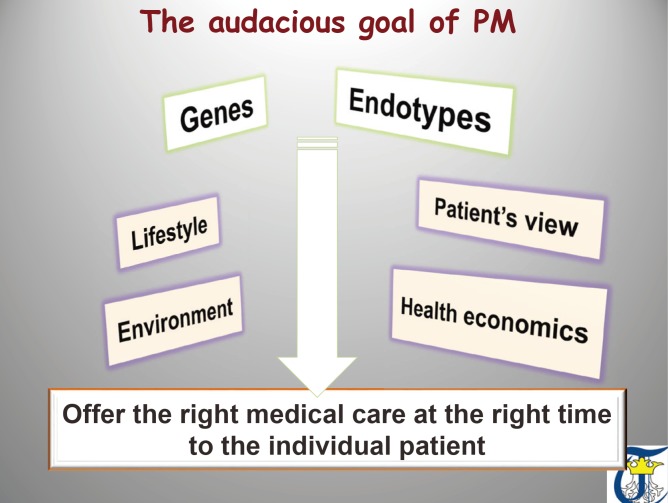
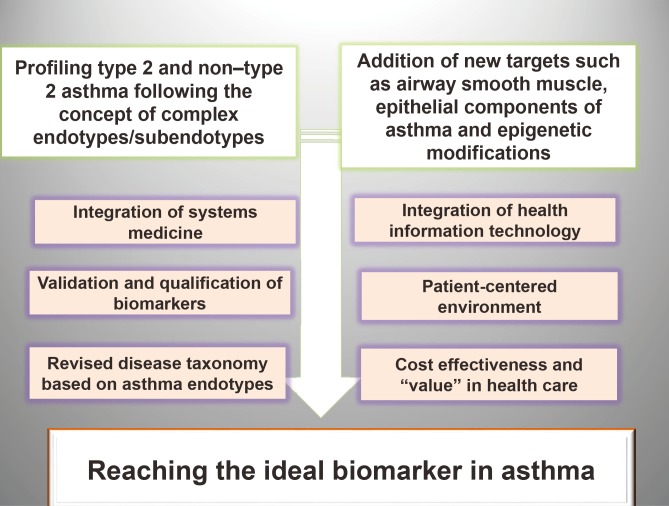
- Measurement of biomarkers has been incorporated within clinical research of asthma to characterize the population and to associate the disease with environmental and therapeutic effects. Regrettably, at present, there are no specific biomarkers, none is validated or qualified, and endotype-driven choices overlap. Biomarkers have not yet reached clinical practice and are not included in current asthma guidelines. Last but not least, the choice of the outcome upholding the value of the biomarkers is extremely difficult, since it has to reflect the mechanistic intervention while being relevant to both the disease and the particular person. On the verge of a new age of asthma healthcare standard, we must embrace and adapt to the key drivers of change. Disease endotypes, biomarkers, and precision medicine represent an emerging model of patient care building on large-scale biologic databases, omics and diverse cellular assays, health information technology, and computational tools for analyzing sizable sets of data. A profound transformation of clinical and research pattern from population to individual risk and from investigator-imposed subjective disease clustering (hypothesis driven) to unbiased, data-driven models is facilitated by the endotype/biomarker-driven approach.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Update on the Management of Nonsteroidal Anti-Inflammatory Drug Hypersensitivity
Wan Yin Winnie Yeung, Hae Sim Park
Yonsei Med J. 2020;61(1):4-14. doi: 10.3349/ymj.2020.61.1.4.Inhaled Corticosteroids and Placebo Treatment Effects in Adult Patients With Cough: A Systematic Review and Meta-analysis
Seung-Eun Lee, Ji-Hyang Lee, Hyun Jung Kim, Byung-Jae Lee, Sang-Heon Cho, David Price, Alyn H. Morice, Woo-Jung Song
Allergy Asthma Immunol Res. 2019;11(6):856-870. doi: 10.4168/aair.2019.11.6.856.Critical Points on the Use of Biologicals in Allergic Diseases and Asthma
Ioana Agache, Catalina Cojanu, Alexandru Laculiceanu, Liliana Rogozea
Allergy Asthma Immunol Res. 2020;12(1):24-41. doi: 10.4168/aair.2020.12.1.24.KAAACI Evidence-Based Clinical Practice Guidelines for Chronic Cough in Adults and Children in Korea
Dae Jin Song, Woo-Jung Song, Jae-Woo Kwon, Gun-Woo Kim, Mi-Ae Kim, Mi-Yeong Kim, Min-Hye Kim, Sang-Ha Kim, Sang-Heon Kim, Sang Hyuck Kim, Sun-Tae Kim, Sae-Hoon Kim, Ja Kyoung Kim, Joo-Hee Kim, Hyun Jung Kim, Hyo-Bin Kim, Kyung-Hee Park, Jae Kyun Yoon, Byung-Jae Lee, Seung-Eun Lee, Young Mok Lee, Yong Ju Lee, Kyung-Hwan Lim, You Hoon Jeon, Eun-Jung Jo, Young-Koo Jee, Hyun Jung Jin, Sun Hee Choi, Gyu Young Hur, Sang-Heon Cho, Sang-Hoon Kim, Dae Hyun Lim
Allergy Asthma Immunol Res. 2018;10(6):591-613. doi: 10.4168/aair.2018.10.6.591.
Reference
-
1. Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015; 372:2229–2234. PMID: 26014593.2. Muraro A, Fokkens WJ, Pietikainen S, Borrelli D, Agache I, Bousquet J, et al. European symposium on precision medicine in allergy and airways diseases: report of the European Union Parliament Symposium (October 14, 2015). Allergy. 2016; 71:583–587. PMID: 26660289.
Article3. Garrod AE. The incidence of alkaptonuria: a study in chemical individuality. Lancet. 1902; 160:1616–1620.
Article4. Muraro A, Lemanske RF Jr, Hellings PW, Akdis CA, Bieber T, Casale TB, et al. Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016; 137:1347–1358. PMID: 27155030.5. Agache I, Akdis CA. Endotypes of allergic diseases and asthma: an important step in building blocks for the future of precision medicine. Allergol Int. 2016; 65:243–252. PMID: 27282212.
Article6. Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008; 372:1107–1119. PMID: 18805339.
Article7. Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012; 67:835–846. PMID: 22594878.
Article8. Lötvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011; 127:355–360. PMID: 21281866.9. Ray A, Oriss TB, Wenzel SE. Emerging molecular phenotypes of asthma. Am J Physiol Lung Cell Mol Physiol. 2015; 308:L130–L140. PMID: 25326577.
Article10. Agache IO. From phenotypes to endotypes to asthma treatment. Curr Opin Allergy Clin Immunol. 2013; 13:249–256. PMID: 23587683.
Article11. Agache I, Sugita K, Morita H, Akdis M, Akdis CA. The complex type 2 endotype in allergy and asthma: from laboratory to bedside. Curr Allergy Asthma Rep. 2015; 15:29. PMID: 26141574.
Article12. European Medicines Agency, Committee for Medicinal Products for Human Use. Zelboraf: summary of opinion. London: European Medicines Agency;2011.13. Maggon K. New drug approvals FDA/EMA in 2010: declining R&D productivity? Knol Publishing Guild (KPG);2011.14. Wagner JA. Overview of biomarkers and surrogate endpoints in drug development. Dis Markers. 2002; 18:41–46. PMID: 12364809.
Article15. Goodsaid FM, Frueh FW, Mattes W. Strategic paths for biomarker qualification. Toxicology. 2008; 245:219–223. PMID: 18280028.
Article16. Agache IO. Endotype driven treatment of asthma. Curr Treat Options Allergy. 2014; 1:198–212.
Article17. U.S. Food and Drug Administration. Guidance for industry: pharmacogenomic data submissions [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;2005. Available from: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm079849.pdf.18. European Medicines Agency. Qualification of novel methodologies for drug development: guidance to applicants [Internet]. London: European Medicines Agency;2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004201.pdf.19. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009; 180:388–395. PMID: 19483109.
Article20. Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J Allergy Clin Immunol. 2011; 127:153–160. 160.e1–160.e9. PMID: 21211650.
Article21. Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, et al. A transcriptome-driven analysis of epithelial brushings and bronchial biopsies to define asthma phenotypes in U-BIOPRED. Am J Respir Crit Care Med. 2017; 195:443–455. PMID: 27580351.
Article22. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007; 104:15858–15863. PMID: 17898169.
Article23. Nicodemus-Johnson J, Naughton KA, Sudi J, Hogarth K, Naurekas ET, Nicolae DL, et al. Genome-wide methylation study identifies an IL-13-induced epigenetic signature in asthmatic airways. Am J Respir Crit Care Med. 2016; 193:376–385. PMID: 26474238.
Article24. Modena BD, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Wu W, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med. 2014; 190:1363–1372. PMID: 25338189.
Article25. Yan X, Chu JH, Gomez J, Koenigs M, Holm C, He X, et al. Noninvasive analysis of the sputum transcriptome discriminates clinical phenotypes of asthma. Am J Respir Crit Care Med. 2015; 191:1116–1125. PMID: 25763605.
Article26. Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015; 191:758–766. PMID: 25611785.
Article27. Agache I, Strasser DS, Klenk A, Agache C, Farine H, Ciobanu C, et al. Serum IL-5 and IL-13 consistently serve as the best predictors for the blood eosinophilia phenotype in adult asthmatics. Allergy. 2016; 71:1192–1202. PMID: 27060452.
Article28. Brasier AR, Victor S, Boetticher G, Ju H, Lee C, Bleecker ER, et al. Molecular phenotyping of severe asthma using pattern recognition of bronchoalveolar lavage-derived cytokines. J Allergy Clin Immunol. 2008; 121:30–37.e6. PMID: 18206505.
Article29. Little FF, Delgado DM, Wexler PJ, Oppenheim FG, Mitchell P, Feldman JA, et al. Salivary inflammatory mediator profiling and correlation to clinical disease markers in asthma. PLoS One. 2014; 9:e84449. PMID: 24409298.
Article30. Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016; 150:789–798. PMID: 27056586.31. Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016; 388:2115–2127. PMID: 27609408.
Article32. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651–659. PMID: 22901886.
Article33. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009; 360:973–984. PMID: 19264686.
Article34. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009; 360:985–993. PMID: 19264687.
Article35. Cabon Y, Molinari N, Marin G, Vachier I, Gamez AS, Chanez P, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy. 2017; 47:129–138. PMID: 27859832.
Article36. Brightling CE, Chanez P, Leigh R, O'Byrne PM, Korn S, She D, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015; 3:692–701. PMID: 26231288.
Article37. Corren J, Lemanske RF Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098. PMID: 21812663.
Article38. Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016; 388:31–44. PMID: 27130691.39. Ledford D, Busse W, Trzaskoma B, Omachi TA, Rosén K, Chipps BE, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2016; Forthcoming.
Article40. Djukanovic R, Hanania N, Busse W, Price D. IgE-mediated asthma: new revelations and future insights. Respir Med. 2016; 112:128–129. PMID: 26577335.
Article41. Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804–811. PMID: 23471469.42. Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007; 370:1422–1431. PMID: 17950857.
Article43. Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012; 130:647–654.e10. PMID: 22857879.
Article44. Kanemitsu Y, Matsumoto H, Mishima M. KiHAC Respiratory Medicine Group. Factors contributing to an accelerated decline in pulmonary function in asthma. Allergol Int. 2014; 63:181–188. PMID: 24759557.
Article45. Matsusaka M, Kabata H, Fukunaga K, Suzuki Y, Masaki K, Mochimaru T, et al. Phenotype of asthma related with high serum periostin levels. Allergol Int. 2015; 64:175–180. PMID: 25838094.
Article46. Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol. 2014; 133:388–394. PMID: 24075231.
Article47. Stephan M, Suhling H, Schade J, Wittlake M, Tasic T, Klemann C, et al. Effects of dipeptidyl peptidase-4 inhibition in an animal model of experimental asthma: a matter of dose, route, and time. Physiol Rep. 2013; 1:e00095. PMID: 24303167.
Article48. Ravensberg AJ, Ricciardolo FL, van Schadewijk A, Rabe KF, Sterk PJ, Hiemstra PS, et al. Eotaxin-2 and eotaxin-3 expression is associated with persistent eosinophilic bronchial inflammation in patients with asthma after allergen challenge. J Allergy Clin Immunol. 2005; 115:779–785. PMID: 15805998.
Article49. Zietkowski Z, Tomasiak MM, Skiepko R, Bodzenta-Lukaszyk A. RANTES in exhaled breath condensate of stable and unstable asthma patients. Respir Med. 2008; 102:1198–1202. PMID: 18603420.
Article50. Parker JM, Oh CK, LaForce C, Miller SD, Pearlman DS, Le C, et al. Safety profile and clinical activity of multiple subcutaneous doses of MEDI-528, a humanized anti-interleukin-9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulm Med. 2011; 11:14. PMID: 21356110.
Article51. Tang W, Smith SG, Salter B, Oliveria JP, Mitchell P, Nusca GM, et al. Allergen-induced increases in interleukin-25 and interleukin-25 receptor expression in mature eosinophils from atopic asthmatics. Int Arch Allergy Immunol. 2016; 170:234–242. PMID: 27685606.
Article52. Yi L, Cheng D, Zhang K, Huo X, Mo Y, Shi H, et al. Intelectin contributes to allergen-induced IL-25, IL-33, and TSLP expression and type 2 response in asthma and atopic dermatitis. Mucosal Immunol. 2017; Forthcoming.
Article53. Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009; 123:472–478. PMID: 19064280.
Article54. Mutalithas K, Guillen C, Day C, Brightling CE, Pavord ID, Wardlaw AJ. CRTH2 expression on T cells in asthma. Clin Exp Immunol. 2010; 161:34–40. PMID: 20491797.
Article55. Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010; 43:305–315. PMID: 19843704.56. Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005; 174:8183–8190. PMID: 15944327.
Article57. Hammad H, Kool M, Soullié T, Narumiya S, Trottein F, Hoogsteden HC, et al. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J Exp Med. 2007; 204:357–367. PMID: 17283205.
Article58. Hoshino M, Nakagawa T, Sano Y, Hirai K. Effect of inhaled corticosteroid on an immunoreactive thymus and activation-regulated chemokine expression in the bronchial biopsies from asthmatics. Allergy. 2005; 60:317–322. PMID: 15679716.
Article59. Mutalithas K, Guillen C, Raport C, Kolbeck R, Soler D, Brightling CE, et al. Expression of CCR8 is increased in asthma. Clin Exp Allergy. 2010; 40:1175–1185. PMID: 20455898.
Article60. Liao SC, Cheng YC, Wang YC, Wang CW, Yang SM, Yu CK, et al. IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J Immunol. 2004; 173:6712–6718. PMID: 15557163.
Article61. Koh YI, Shim JU. Association between sputum natural killer T cells and eosinophilic airway inflammation in human asthma. Int Arch Allergy Immunol. 2010; 153:239–248. PMID: 20484922.
Article62. Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009; 183:5094–5103. PMID: 19801525.
Article63. Ueno T, Miyazaki E, Ando M, Nureki S, Kumamoto T. Osteopontin levels are elevated in patients with eosinophilic pneumonia. Respirology. 2010; 15:1111–1121. PMID: 20796249.
Article64. Gangwar RS, Minai-Fleminger Y, Seaf M, Gutgold A, Shikotra A, Barber C, et al. CD48 on blood leukocytes and in serum of asthma patients varies with severity. Allergy. 2016; Forthcoming.
Article65. Grotta MB, Squebola-Cola DM, Toro AA, Ribeiro MA, Mazon SB, Ribeiro JD, et al. Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm Med. 2013; 13:39. PMID: 23773659.
Article66. Curran CS, Bertics PJ. Lactoferrin regulates an axis involving CD11b and CD49d integrins and the chemokines MIP-1α and MCP-1 in GM-CSF-treated human primary eosinophils. J Interferon Cytokine Res. 2012; 32:450–461. PMID: 22731992.
Article67. Kelly EA, Koziol-White CJ, Clay KJ, Liu LY, Bates ME, Bertics PJ, et al. Potential contribution of IL-7 to allergen-induced eosinophilic airway inflammation in asthma. J Immunol. 2009; 182:1404–1410. PMID: 19155487.
Article68. Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, et al. ICOS: ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015; 42:538–551. PMID: 25769613.69. Fang P, Zhou L, Zhou Y, Kolls JK, Zheng T, Zhu Z. Immune modulatory effects of IL-22 on allergen-induced pulmonary inflammation. PLoS One. 2014; 9:e107454. PMID: 25254361.
Article70. Hartwig C, Munder A, Glage S, Wedekind D, Schenk H, Seifert R, et al. The histamine H4-receptor (H4 R) regulates eosinophilic inflammation in ovalbumin-induced experimental allergic asthma in mice. Eur J Immunol. 2015; 45:1129–1140. PMID: 25501767.71. Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014; 134:671–678.e4. PMID: 25171868.
Article72. Gunawardhana LP, Gibson PG, Simpson JL, Benton MC, Lea RA, Baines KJ. Characteristic DNA methylation profiles in peripheral blood monocytes are associated with inflammatory phenotypes of asthma. Epigenetics. 2014; 9:1302–1316. PMID: 25147914.
Article73. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006; 11:54–61. PMID: 16423202.
Article74. Agache I. Non-eosinophilic asthma endotypes. Curr Treat Options Allergy. 2015; 2:257–267.
Article75. Li Q, Baines KJ, Gibson PG, Wood LG. Changes in expression of genes regulating airway inflammation following a high-fat mixed meal in asthmatics. Nutrients. 2016; 8:E30. PMID: 26751474.
Article76. Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010; 104:1131–1137. PMID: 20338742.
Article77. Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001; 119:1329–1336. PMID: 11348936.78. Hicks A, Goodnow R Jr, Cavallo G, Tannu SA, Ventre JD, Lavelle D, et al. Effects of LTB4 receptor antagonism on pulmonary inflammation in rodents and non-human primates. Prostaglandins Other Lipid Mediat. 2010; 92:33–43. PMID: 20214997.
Article79. Wood LG, Simpson JL, Hansbro PM, Gibson PG. Potentially pathogenic bacteria cultured from the sputum of stable asthmatics are associated with increased 8-isoprostane and airway neutrophilia. Free Radic Res. 2010; 44:146–154. PMID: 19922242.
Article80. Bogaert P, Naessens T, De Koker S, Hennuy B, Hacha J, Smet M, et al. Inflammatory signatures for eosinophilic vs. neutrophilic allergic pulmonary inflammation reveal critical regulatory checkpoints. Am J Physiol Lung Cell Mol Physiol. 2011; 300:L679–L690. PMID: 21335522.
Article81. Roussel L, Houle F, Chan C, Yao Y, Bérubé J, Olivenstein R, et al. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 2010; 184:4531–4537. PMID: 20228195.
Article82. Hsia BJ, Whitehead GS, Thomas SY, Nakano K, Gowdy KM, Aloor JJ, et al. Trif-dependent induction of Th17 immunity by lung dendritic cells. Mucosal Immunol. 2015; 8:186–197. PMID: 24985082.
Article83. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006; 7:135. PMID: 17083726.
Article84. Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013; 188:1294–1302. PMID: 24200404.
Article85. Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010; 125:1028–1036.e13. PMID: 20398920.
Article86. Kikuchi S, Kikuchi I, Takaku Y, Kobayashi T, Hagiwara K, Kanazawa M, et al. Neutrophilic inflammation and CXC chemokines in patients with refractory asthma. Int Arch Allergy Immunol. 2009; 149(Suppl 1):87–93. PMID: 19494512.
Article87. Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010; 31:318–324. PMID: 20620114.
Article88. Seys SF, Hox V, Van Gerven L, Dilissen E, Marijsse G, Peeters E, et al. Damage-associated molecular pattern and innate cytokine release in the airways of competitive swimmers. Allergy. 2015; 70:187–194. PMID: 25358760.
Article89. Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007; 178:8148–8157. PMID: 17548653.
Article90. Gao P, Gibson PG, Baines KJ, Yang IA, Upham JW, Reynolds PN, et al. Anti-inflammatory deficiencies in neutrophilic asthma: reduced galectin-3 and IL-1RA/IL-1β. Respir Res. 2015; 16:5. PMID: 25616863.
Article91. Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in amish and Hutterite farm children. N Engl J Med. 2016; 375:411–421. PMID: 27518660.
Article92. Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlén SE, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009; 179:549–558. PMID: 19136369.93. Holgate ST, Noonan M, Chanez P, Busse W, Dupont L, Pavord I, et al. Efficacy and safety of etanercept in moderate-to-severe asthma: a randomised, controlled trial. Eur Respir J. 2011; 37:1352–1359. PMID: 21109557.
Article94. Bullens DM, Decraene A, Dilissen E, Meyts I, De Boeck K, Dupont LJ, et al. Type III IFN-lambda mRNA expression in sputum of adult and school-aged asthmatics. Clin Exp Allergy. 2008; 38:1459–1467. PMID: 18564328.95. Djukanović R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, et al. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med. 2014; 190:145–154. PMID: 24937476.
Article96. Matangkasombut P, Marigowda G, Ervine A, Idris L, Pichavant M, Kim HY, et al. Natural killer T cells in the lungs of patients with asthma. J Allergy Clin Immunol. 2009; 123:1181–1185. PMID: 19356791.
Article97. Chun E, Lee SH, Lee SY, Shim EJ, Cho SH, Min KU, et al. Toll-like receptor expression on peripheral blood mononuclear cells in asthmatics; implications for asthma management. J Clin Immunol. 2010; 30:459–464. PMID: 20072849.
Article98. Månsson Kvarnhammar A, Tengroth L, Adner M, Cardell LO. Innate immune receptors in human airway smooth muscle cells: activation by TLR1/2, TLR3, TLR4, TLR7 and NOD1 agonists. PLoS One. 2013; 8:e68701. PMID: 23861935.
Article99. Møller-Larsen S, Nyegaard M, Haagerup A, Vestbo J, Kruse TA, Børglum AD. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax. 2008; 63:1064–1069. PMID: 18682521.
Article100. Roponen M, Yerkovich ST, Hollams E, Sly PD, Holt PG, Upham JW. Toll-like receptor 7 function is reduced in adolescents with asthma. Eur Respir J. 2010; 35:64–71. PMID: 19643938.
Article101. Wood LG, Simpson JL, Wark PA, Powell H, Gibson PG. Characterization of innate immune signalling receptors in virus-induced acute asthma. Clin Exp Allergy. 2011; 41:640–648. PMID: 21129050.
Article102. Denlinger LC, Shi L, Guadarrama A, Schell K, Green D, Morrin A, et al. Attenuated P2X7 pore function as a risk factor for virus-induced loss of asthma control. Am J Respir Crit Care Med. 2009; 179:265–270. PMID: 19201928.103. Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007; 357:2016–2027. PMID: 18003958.
Article104. Tang H, Fang Z, Sun Y, Li B, Shi Z, Chen J, et al. YKL-40 in asthmatic patients, and its correlations with exacerbation, eosinophils and immunoglobulin E. Eur Respir J. 2010; 35:757–760. PMID: 20356987.
Article105. Koponen P, He Q, Helminen M, Nuolivirta K, Korppi M. Association of MBL2 polymorphism with asthma after bronchiolitis in infancy. Pediatr Int. 2012; 54:619–622. PMID: 22512728.106. Baines KJ, Wright TK, Simpson JL, McDonald VM, Wood LG, Parsons KS, et al. Airway β-defensin-1 protein is elevated in COPD and severe asthma. Mediators Inflamm. 2015; 2015:407271. PMID: 26568662.107. Bratke K, Klug A, Julius P, Kuepper M, Lommatzsch M, Sparmann G, et al. Granzyme K: a novel mediator in acute airway inflammation. Thorax. 2008; 63:1006–1011. PMID: 18559365.
Article108. Xiao Y, Motomura S, Deyev V, Podack ER. TNF superfamily member 13, APRIL, inhibits allergic lung inflammation. Eur J Immunol. 2011; 41:164–171. PMID: 21182087.
Article109. Croteau-Chonka DC, Qiu W, Martinez FD, Strunk RC, Lemanske RF Jr, Liu AH, et al. Gene expression profiling in blood provides reproducible molecular insights into asthma control. Am J Respir Crit Care Med. 2017; 195:179–188. PMID: 27494826.
Article110. Mackay RM, Grainge CL, Lau LC, Barber C, Clark HW, Howarth PH. Airway surfactant protein d deficiency in adults with severe asthma. Chest. 2016; 149:1165–1172. PMID: 26836907.111. Kazani S, Planaguma A, Ono E, Bonini M, Zahid M, Marigowda G, et al. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. J Allergy Clin Immunol. 2013; 132:547–553. PMID: 23608729.
Article112. Planagumà A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008; 178:574–582. PMID: 18583575.113. Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, et al. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005; 115:55–60. PMID: 15637547.
Article114. Dauletbaev N, Lands LC. Could relative abundance of airway lipoxins be the clue to restore corticosteroid sensitivity in severe asthma? J Allergy Clin Immunol. 2016; 137:1807–1808. PMID: 27084402.
Article115. Gagliardo R, Gras D, La Grutta S, Chanez P, Di Sano C, Albano GD, et al. Airway lipoxin A4/formyl peptide receptor 2-lipoxin receptor levels in pediatric patients with severe asthma. J Allergy Clin Immunol. 2016; 137:1796–1806. PMID: 26971688.
Article116. Chung KF. Lipoxins and epoxyeicosatrienoic acids. Potential for inhibitors of soluble epoxide hydrolase in severe asthma? Am J Respir Crit Care Med. 2014; 190:848–850. PMID: 25317461.
Article117. Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007; 178:496–502. PMID: 17182589.
Article118. Gu Z, Lamont GJ, Lamont RJ, Uriarte SM, Wang H, Scott DA. Resolvin D1, resolvin D2 and maresin 1 activate the GSK3β anti-inflammatory axis in TLR4-engaged human monocytes. Innate Immun. 2016; 22:186–195. PMID: 26878867.
Article119. Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, et al. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008; 367:509–515. PMID: 18190790.
Article120. Aoki H, Hisada T, Ishizuka T, Utsugi M, Ono A, Koga Y, et al. Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochem Biophys Res Commun. 2010; 400:128–133. PMID: 20708601.
Article121. Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008; 9:873–879. PMID: 18568027.122. Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J Immunol. 2011; 186:6129–6135. PMID: 21515793.
Article123. Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009; 206:15–23. PMID: 19103881.
Article124. Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011; 128:549–556. 556.e1–556.e12. PMID: 21752437.
Article125. de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008; 86:105–112. PMID: 18418437.
Article126. Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, Rückert B, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017; 139:93–103. PMID: 27312821.
Article127. Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007; 356:1327–1337. PMID: 17392302.
Article128. Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010; 181:116–124. PMID: 19815809.129. Doeing DC, Husain AN, Naureckas ET, White SR, Hogarth DK. Bronchial thermoplasty failure in severe persistent asthma: a case report. J Asthma. 2013; 50:799–801. PMID: 23651158.
Article130. Wilson SJ, Ward JA, Sousa AR, Corfield J, Bansal AT, De Meulder B, et al. Severe asthma exists despite suppressed tissue inflammation: findings of the U-BIOPRED study. Eur Respir J. 2016; 48:1307–1319. PMID: 27799384.
Article131. Hoshino M, Ohtawa J, Akitsu K. Association of airway wall thickness with serum periostin in steroid-naive asthma. Allergy Asthma Proc. 2016; 37:225–230. PMID: 27178891.
Article132. Lee YM, Park JS, Hwang JH, Park SW, Uh ST, Kim YH, et al. High-resolution CT findings in patients with near-fatal asthma: comparison of patients with mild-to-severe asthma and normal control subjects and changes in airway abnormalities following steroid treatment. Chest. 2004; 126:1840–1848. PMID: 15596682.133. Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest. 2008; 134:1183–1191. PMID: 18641116.
Article134. Gonem S, Hardy S, Buhl N, Hartley R, Soares M, Kay R, et al. Characterization of acinar airspace involvement in asthmatic patients by using inert gas washout and hyperpolarized (3)helium magnetic resonance. J Allergy Clin Immunol. 2016; 137:417–425. PMID: 26242298.
Article135. Ueda T, Niimi A, Matsumoto H, Takemura M, Hirai T, Yamaguchi M, et al. Role of small airways in asthma: investigation using high-resolution computed tomography. J Allergy Clin Immunol. 2006; 118:1019–1025. PMID: 17088124.
Article136. Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest. 2013; 143:1436–1443. PMID: 23648907.137. Gagliardo R, La Grutta S, Chanez P, Profita M, Paternò A, Cibella F, et al. Non-invasive markers of airway inflammation and remodeling in childhood asthma. Pediatr Allergy Immunol. 2009; 20:780–790. PMID: 19788537.
Article138. Dolhnikoff M, da Silva LF, de Araujo BB, Gomes HA, Fernezlian S, Mulder A, et al. The outer wall of small airways is a major site of remodeling in fatal asthma. J Allergy Clin Immunol. 2009; 123:1090–1097.e1. PMID: 19361849.
Article139. Obase Y, Rytilä P, Metso T, Pelkonen AS, Tervahartiala T, Turpeinen M, et al. Effects of inhaled corticosteroids on metalloproteinase-8 and tissue inhibitor of metalloproteinase-1 in the airways of asthmatic children. Int Arch Allergy Immunol. 2010; 151:247–254. PMID: 19786805.
Article140. Mukhopadhyay S, Sypek J, Tavendale R, Gartner U, Winter J, Li W, et al. Matrix metalloproteinase-12 is a therapeutic target for asthma in children and young adults. J Allergy Clin Immunol. 2010; 126:70–76.e16. PMID: 20546881.
Article141. Chen J, Deng L, Dreymüller D, Jiang X, Long J, Duan Y, et al. A novel peptide ADAM8 inhibitor attenuates bronchial hyperresponsiveness and Th2 cytokine mediated inflammation of murine asthmatic models. Sci Rep. 2016; 6:30451. PMID: 27458083.
Article142. Foley SC, Mogas AK, Olivenstein R, Fiset PO, Chakir J, Bourbeau J, et al. Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J Allergy Clin Immunol. 2007; 119:863–871. PMID: 17339047.
Article143. Jongepier H, Boezen HM, Dijkstra A, Howard TD, Vonk JM, Koppelman GH, et al. Polymorphisms of the ADAM33 gene are associated with accelerated lung function decline in asthma. Clin Exp Allergy. 2004; 34:757–760. PMID: 15144468.
Article144. Kim SH, Pei QM, Jiang P, Yang M, Qian XJ, Liu JB. Effect of active vitamin D3 on VEGF-induced ADAM33 expression and proliferation in human airway smooth muscle cells: implications for asthma treatment. Respir Res. 2017; 18:7. PMID: 28056993.
Article145. Schedel M, Depner M, Schoen C, Weiland SK, Vogelberg C, Niggemann B, et al. The role of polymorphisms in ADAM33, a disintegrin and metalloprotease 33, in childhood asthma and lung function in two German populations. Respir Res. 2006; 7:91. PMID: 16784537.
Article146. Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy. 2010; 65:946–958. PMID: 20415716.
Article147. Fujita H, Chalubinski M, Rhyner C, Indermitte P, Meyer N, Ferstl R, et al. Claudin-1 expression in airway smooth muscle exacerbates airway remodeling in asthmatic subjects. J Allergy Clin Immunol. 2011; 127:1612–1621.e8. PMID: 21624620.
Article148. Liu G, Cooley MA, Jarnicki AG, Hsu AC, Nair PM, Haw TJ, et al. Fibulin-1 regulates the pathogenesis of tissue remodeling in respiratory diseases. JCI Insight. 2016; 1:e86380. PMID: 27398409.
Article149. Lau JY, Oliver BG, Baraket M, Beckett EL, Hansbro NG, Moir LM, et al. Fibulin-1 is increased in asthma--a novel mediator of airway remodeling? PLoS One. 2010; 5:e13360. PMID: 20967215.150. Druilhe A, Zahm JM, Benayoun L, El Mehdi D, Grandsaigne M, Dombret MC, et al. Epithelium expression and function of retinoid receptors in asthma. Am J Respir Cell Mol Biol. 2008; 38:276–282. PMID: 17884991.
Article151. Grainge C, Dulay V, Ward J, Sammut D, Davies E, Green B, et al. Resistin-like molecule-β is induced following bronchoconstriction of asthmatic airways. Respirology. 2012; 17:1094–1100. PMID: 22758223.
Article152. James AJ, Reinius LE, Verhoek M, Gomes A, Kupczyk M, Hammar U, et al. Increased YKL-40 and chitotriosidase in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016; 193:131–142. PMID: 26372680.
Article153. Samitas K, Poulos N, Semitekolou M, Morianos I, Tousa S, Economidou E, et al. Activin-A is overexpressed in severe asthma and is implicated in angiogenic processes. Eur Respir J. 2016; 47:769–782. PMID: 26869672.
Article154. Simoes DC, Xanthou G, Petrochilou K, Panoutsakopoulou V, Roussos C, Gratziou C. Osteopontin deficiency protects against airway remodeling and hyperresponsiveness in chronic asthma. Am J Respir Crit Care Med. 2009; 179:894–902. PMID: 19234104.
Article155. van Staa TP, Goldacre B, Buchan I, Smeeth L. Big health data: the need to earn public trust. BMJ. 2016; 354:i3636. PMID: 27418128.
Article156. Schneeweiss S. Learning from big health care data. N Engl J Med. 2014; 370:2161–2163. PMID: 24897079.
Article157. Williams SM, Moore JH. Big data analysis on autopilot? BioData Min. 2013; 6:22. PMID: 24314297.
Article158. Chaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006; 144:742–752. PMID: 16702590.
Article159. Blumenthal D. Stimulating the adoption of health information technology. N Engl J Med. 2009; 360:1477–1479. PMID: 19321856.
Article160. Gibson CJ, Dixon BE, Abrams K. Convergent evolution of health information management and health informatics: a perspective on the future of information professionals in health care. Appl Clin Inform. 2015; 6:163–184. PMID: 25848421.161. Milgrom H, Tran ZV. The rise of health information technology. Curr Opin Allergy Clin Immunol. 2010; 10:178–180. PMID: 20386434.
Article162. Howard R, Rattray M, Prosperi M, Custovic A. Distinguishing asthma phenotypes using machine learning approaches. Curr Allergy Asthma Rep. 2015; 15:38. PMID: 26143394.
Article163. Custovic A, Ainsworth J, Arshad H, Bishop C, Buchan I, Cullinan P, et al. The Study Team for Early Life Asthma Research (STELAR) consortium ‘Asthma e-lab’: team science bringing data, methods and investigators together. Thorax. 2015; 70:799–801. PMID: 25805205.
Article164. Matui P, Wyatt JC, Pinnock H, Sheikh A, McLean S. Computer decision support systems for asthma: a systematic review. NPJ Prim Care Respir Med. 2014; 24:14005. PMID: 24841952.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biomarkers of adult asthma and personalized medicine
- Effective Strategies for Managing Asthma Exacerbations for Precision Medicine
- Biologic agents for asthma treatment
- Biomarkers in Chronic Rhinosinusitis with Nasal Polyp: Personalized Medicine Based on Endotype
- Phenotypes and endotypes of atopic dermatitis: Clinical implications