Clin Exp Vaccine Res.
2016 Jul;5(2):175-178. 10.7774/cevr.2016.5.2.175.
A Bordetella pertussis proteoliposome induces protection in mice without affecting the immunogenicity of diphtheria and tetanus toxoids in a trivalent formulation
- Affiliations
-
- 1Finlay Institute, Havana, Cuba. sfernandez@finlay.edu.cu
- 2Medical Faculty and Institute of Basic and Preclinical Science "Victoria de Girón", University of Medical Sciences of Havana, Havana, Cuba.
- KMID: 2421565
- DOI: http://doi.org/10.7774/cevr.2016.5.2.175
Abstract
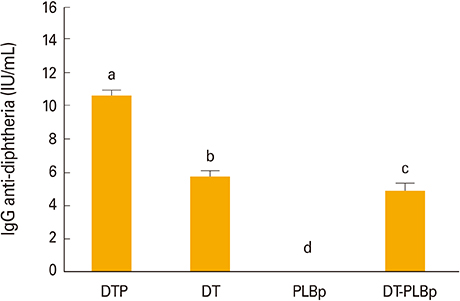
- In this study, a formulation of Bordetella pertussis proteoliposome (PLBp), diphtheria, and tetanus toxoids and alum (DT-PLBp) was evaluated as a trivalent vaccine candidate in BALB/c mice. Vaccine-induced protection was estimated using the intranasal challenge for pertussis and enzyme-linked immunosorbent assay fvto assess serological responses for diphtheria or tetanus. Both, diphtheria-tetanus-whole cell pertussis (DTP) and diphtheria-tetanus vaccines (DT) were used as controls. Animals immunized with DT-PLBp, PLBp alone, and DTP showed total reduction of CFU in lungs 7 days after intranasal challenge. Likewise, formulations DT-PLBp, DTP, and DT elicited antibody levels ≥2 IU/mL against tetanus and diphtheria, considered protective when neutralization tests are used. Overall, results showed that combination of PLBp with tetanus and diphtheria toxoids did not affect the immunogenicity of each antigen alone.
Keyword
MeSH Terms
Figure
Reference
-
1. Pertussis vaccines: WHO position paper. Wkly Epidemiol Rec. 2010; 85:385–400.2. Cherry JD. Why do pertussis vaccines fail? Pediatrics. 2012; 129:968–970.
Article3. Storsaeter J, Wolter J. Is there a need for a new generation of vaccines against pertussis? Expert Opin Emerg Drugs. 2006; 11:195–205.
Article4. Meade BD, Plotkin SA, Locht C. Possible options for new pertussis vaccines. J Infect Dis. 2014; 209:Suppl 1. S24–S27.
Article5. Acevedo R, Fernandez S, Zayas C, et al. Bacterial outer membrane vesicles and vaccine applications. Front Immunol. 2014; 5:121.
Article6. Sierra GV, Campa HC, Varcacel NM, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991; 14:195–207.7. Fernandez S, Fajardo EM, Mandiarote A, et al. A proteoliposome formulation derived from Bordetella pertussis induces protection in two murine challenge models. BMC Immunol. 2013; 14:Suppl 1. S8.
Article8. Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005; 18:326–382.
Article9. Chovel ML, Mandiarote A, Ontivero I, et al. Development of 3Rs alternatives for determining potency and toxicity of vaccines in Cuba: current challenges and research projects in progress. ALTEX. 2012; 29:71–76.10. Guiso N, Capiau C, Carletti G, Poolman J, Hauser P. Intranasal murine model of Bordetella pertussis infection. I. Prediction of protection in human infants by acellular vaccines. Vaccine. 1999; 17:2366–2376.
Article11. WHO/UNICEF coverage estimates 2012 revision, July 2013. 194 WHO Member States. Map production: immunization vaccines and biologicals (IVB). Geneva: World Health Organization;2013.12. Forsyth KD, Wirsing von Konig CH, Tan T, Caro J, Plotkin S. Prevention of pertussis: recommendations derived from the second Global Pertussis Initiative roundtable meeting. Vaccine. 2007; 25:2634–2642.
Article13. Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation: two sides of the same coin. Epidemiol Infect. 2014; 142:685–694.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preliminary study on the immunogenicity of a newly developed GCC Tdap vaccine and its protection efficacy against Bordetella pertussis in a murine intranasal challenge model
- Tetanus–diphtheria–acellular pertussis vaccination for adults: an update
- Evaluation of Potency on Diphtheria and Tetanus Toxoid for Adult Vaccines by In Vivo Toxin Neutralization Assay Using National Reference Standards
- Rates of Adverse Reactions Associated with Modified DPT Vaccine in Korean Infants and Children
- New DTaP Vaccine