J Breast Cancer.
2018 Sep;21(3):251-258. 10.4048/jbc.2018.21.e40.
Hsa-miRNA-143-3p Reverses Multidrug Resistance of Triple-Negative Breast Cancer by Inhibiting the Expression of Its Target Protein Cytokine-Induced Apoptosis Inhibitor 1 In Vivo
- Affiliations
-
- 1Department of Medical Oncology, Second Affiliated Hospital of Harbin Medical University, Harbin, China. doctorlu1972@163.com
- KMID: 2421365
- DOI: http://doi.org/10.4048/jbc.2018.21.e40
Abstract
- PURPOSE
Multidrug resistance (MDR) remains a major obstacle in the treatment of triple-negative breast cancer (TNBC) with conventional chemotherapeutic agents. A previous study demonstrated that hsa-miRNA-143-3p plays a vital role in drug resistance of TNBC. Downregulation of hsa-miRNA-143-3p upregulated the expression of its target protein cytokine-induced apoptosis inhibitor 1 (CIAPIN1) in order to activate MDR, while upregulation of hsa-miRNA-143-3p effectively enhances the sensitivity of drug-resistant TNBC cells to chemotherapeutics. The present study aimed to further verify these findings in vivo.
METHODS
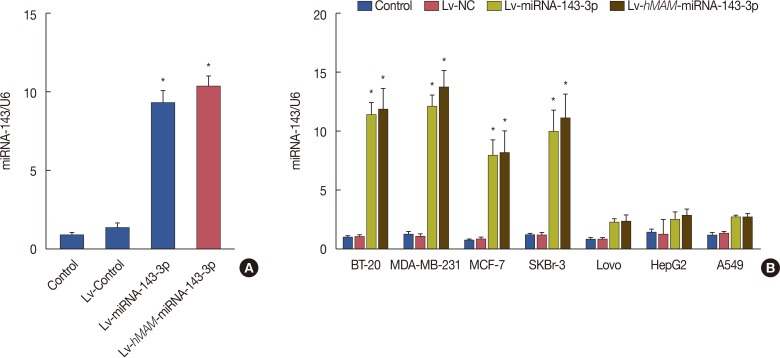
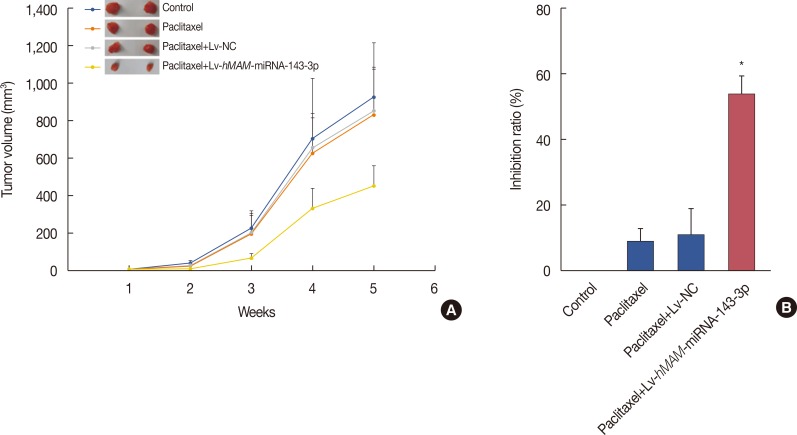
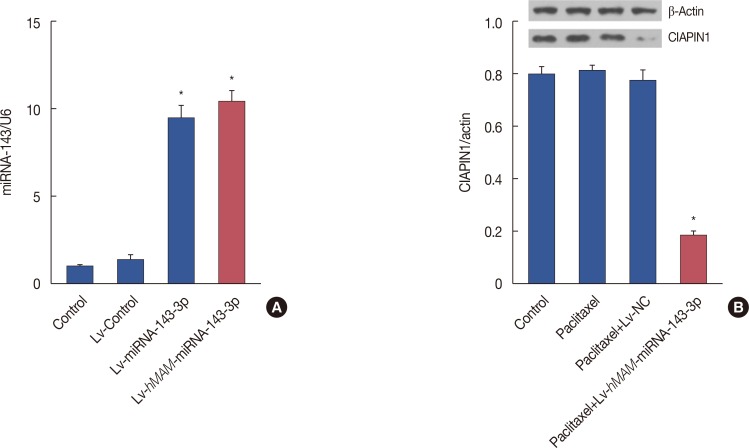
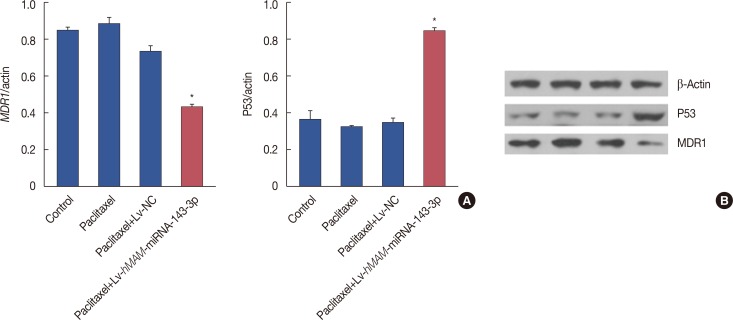
We established a hypodermic tumor nude mice model using paclitaxel-resistant TNBC cells. We expressed ectopic hsa-miRNA-143-3p under the control of a breast cancer-specific human mammaglobin promoter that guided the efficient expression of exogenous hsa-miRNA-143-3p only in breast cancer cells. Thereafter, we overexpressed hsa-miRNA-143-3p in xenografts using a recombinant virus system and quantified the expression of hsa-miRNA-143-3p, CIAPIN1 protein, and proteins encoded by related functional genes by western blot.
RESULTS
We successfully completed the prospective exploration of the intravenous virus injection pattern from extensive expression to targeted expression. The overexpression of hsa-miRNA-143-3p significantly alleviated chemoresistance of TNBC by inhibiting viability. In addition, we observed that the expression of CIAPIN1 as a hsa-miRNA-143-3p target protein was remarkably decreased.
CONCLUSION
We partly illustrated the mechanism underlying the hsa-miRNA-143-3p/CIAPIN1 drug resistance pathway. HsamiRNA-143-3p as a tumor suppressive microRNA may be a novel target to effectively reverse MDR of TNBC in vivo.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhou S, Sun X, Yu L, Zhou R, Li A, Li M, et al. Differential expression and clinical significance of epithelial-mesenchymal transition markers among different histological types of triple-negative breast cancer. J Cancer. 2018; 9:604–613. PMID: 29483966.
Article2. Yardley DA, Arrowsmith ER, Daniel BR, Eakle J, Brufsky A, Drosick DR, et al. TITAN: phase III study of doxorubicin/cyclophosphamide followed by ixabepilone or paclitaxel in early-stage triple-negative breast cancer. Breast Cancer Res Treat. 2017; 164:649–658. PMID: 28508185.
Article3. Doddapaneni R, Patel K, Chowdhury N, Singh M. Reversal of drug-resistance by noscapine chemo-sensitization in docetaxel resistant triple negative breast cancer. Sci Rep. 2017; 7:15824. PMID: 29158480.
Article4. Vaidyanathan A, Sawers L, Gannon AL, Chakravarty P, Scott AL, Bray SE, et al. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br J Cancer. 2016; 115:431–441. PMID: 27415012.
Article5. Yang CH, Wang C, Ojima I, Horwitz SB. Taxol analogues exhibit differential effects on photoaffinity labeling of beta-tubulin and the multidrug resistance associated P-glycoprotein. J Nat Prod. 2018; 81:600–606. PMID: 29517223.6. Ramassone A, Pagotto S, Veronese A, Visone R. Epigenetics and microRNAs in cancer. Int J Mol Sci. 2018; 19:pii: E459.
Article7. Bach DH, Hong JY, Park HJ, Lee SK. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int J Cancer. 2017; 141:220–230. PMID: 28240776.
Article8. Zhou J, Wu S, Chen Y, Zhao J, Zhang K, Wang J, et al. MicroRNA-143 is associated with the survival of ALDH1+CD133+ osteosarcoma cells and the chemoresistance of osteosarcoma. Exp Biol Med (Maywood). 2015; 240:867–875. PMID: 25576341.
Article9. Zhuang M, Shi Q, Zhang X, Ding Y, Shan L, Shan X, et al. Involvement of miR-143 in cisplatin resistance of gastric cancer cells via targeting IGF1R and BCL2. Tumour Biol. 2015; 36:2737–2745. PMID: 25492481.
Article10. Simmer F, Venderbosch S, Dijkstra JR, Vink-Börger EM, Faber C, Mekenkamp LJ, et al. MicroRNA-143 is a putative predictive factor for the response to fluoropyrimidine-based chemotherapy in patients with metastatic colorectal cancer. Oncotarget. 2015; 6:22996–23007. PMID: 26392389.
Article11. Wang H, Li Q, Niu X, Wang G, Zheng S, Fu G, et al. miR-143 inhibits bladder cancer cell proliferation and enhances their sensitivity to gemcitabine by repressing IGF-1R signaling. Oncol Lett. 2017; 13:435–440. PMID: 28123579.
Article12. Huang Z, Su GF, Hu WJ, Bi XX, Zhang L, Wan G. The study on expression of CIAPIN1 interfering hepatocellular carcinoma cell proliferation and its mechanisms. Eur Rev Med Pharmacol Sci. 2017; 21:3054–3060. PMID: 28742201.13. Zhang YF, Li XH, Shi YQ, Wu YY, Li N, He Q, et al. CIAPIN1 confers multidrug resistance through up-regulation of MDR-1 and Bcl-L in LoVo/Adr cells and is independent of p53. Oncol Rep. 2011; 25:1091–1098. PMID: 21240465.
Article14. Zhang XW, Liu L, Zhang XZ, Bo P. Kanglaite inhibits the expression of drug resistance genes through suppressing PVT1 in cisplatin-resistant gastric cancer cells. Exp Ther Med. 2017; 14:1789–1794. PMID: 28810651.
Article15. Wang J, Li Q, Wang C, Xiong Q, Lin Y, Sun Q, et al. Knock-down of CIAPIN1 sensitizes K562 chronic myeloid leukemia cells to Imatinib by regulation of cell cycle and apoptosis-associated members via NF-kappaB and ERK5 signaling pathway. Biochem Pharmacol. 2016; 99:132–145. PMID: 26679828.16. Wang XM, Gao SJ, Guo XF, Sun WJ, Yan ZQ, Wang WX, et al. CIAPIN1 gene silencing enhances chemosensitivity in a drug-resistant animal model in vivo. Braz J Med Biol Res. 2014; 47:273–278. PMID: 24676475.
Article17. Wang JH, Wang XW, Qu D, Sun JW, Guo FX, Lu D. Upregulation of microRNA-143 reverses drug resistance in human breast cancer cells via inhibition of cytokine-induced apoptosis inhibitor 1. Oncol Lett. 2017; 13:4695–4700. PMID: 28588724.
Article18. Chen D, Si W, Shen J, Du C, Lou W, Bao C, et al. miR-27b-3p inhibits proliferation and potentially reverses multi-chemoresistance by targeting CBLB/GRB2 in breast cancer cells. Cell Death Dis. 2018; 9:188. PMID: 29416005.
Article19. Kopczyńska E. Role of microRNAs in the resistance of prostate cancer to docetaxel and paclitaxel. Contemp Oncol (Pozn). 2015; 19:423–427. PMID: 26843836.
Article20. Cao S, Lin L, Xia X, Wu H. MicroRNA-761 promotes the sensitivity of colorectal cancer cells to 5-fluorouracil through targeting FOXM1. Oncotarget. 2017; 9:321–331. PMID: 29416616.
Article21. Cao W, Wei W, Zhan Z, Xie D, Xie Y, Xiao Q. Regulation of drug resistance and metastasis of gastric cancer cells via the microRNA647-ANK2 axis. Int J Mol Med. 2018; 41:1958–1966. PMID: 29328428.
Article22. Huang G, Pan J, Ye Z, Fang B, Cheng W, Cao Z. Overexpression of miR-216b sensitizes NSCLC cells to cisplatin-induced apoptosis by targeting c-Jun. Oncotarget. 2017; 8:104206–104215. PMID: 29262633.
Article23. Sharon D, Kamen A. Advancements in the design and scalable production of viral gene transfer vectors. Biotechnol Bioeng. 2018; 115:25–40. PMID: 28941274.
Article24. Lukashev AN, Zamyatnin AA Jr. Viral vectors for gene therapy: current state and clinical perspectives. Biochemistry (Mosc). 2016; 81:700–708. PMID: 27449616.
Article25. Li C, Zhang T. Human mammaglobin: a specific marker for breast cancer prognosis. J BUON. 2016; 21:35–41. PMID: 27061528.26. Xue C, Wang C, Sun Y, Meng Q, Liu Z, Huo X, et al. Targeting P-glycoprotein function, p53 and energy metabolism: combination of metformin and 2-deoxyglucose reverses the multidrug resistance of MCF-7/Dox cells to doxorubicin. Oncotarget. 2017; 8:8622–8632. PMID: 28052008.
Article27. Gameiro M, Silva R, Rocha-Pereira C, Carmo H, Carvalho F, Bastos ML, et al. Cellular models and in vitro assays for the screening of modulators of P-gp, MRP1 and BCRP. Molecules. 2017; 22:pii: E600.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The MicroRNA hsa-let-7g Promotes Proliferation and Inhibits Apoptosis in Lung Cancer by Targeting HOXB1
- LncRNA XIST promotes carboplatin resistance of ovarian cancer through activating autophagy via targeting miR-506-3p/FOXP1 axis
- MiR-103a-3p Contributes to the Progression of Colorectal Cancer by Regulating GREM2 Expression
- MiR-338-3p Enhances Ovarian Cancer Cell Sensitivity to Cisplatin by Downregulating WNT2B
- Upregulation of MicroRNA-1246 Is Associated with BRAF Inhibitor Resistance in Melanoma Cells with Mutant BRAF