J Vet Sci.
2018 Sep;19(5):620-626. 10.4142/jvs.2018.19.5.620.
Detection of progressive and regressive phase and LINE-1 retrotransposon in transfected dogs with transmissible venereal tumor during chemotherapy
- Affiliations
-
- 1Department of Pathology, Faculty of Veterinary Medicine, Ankara University, 06110 Ankara, Turkey. sevilvural@yahoo.com
- 2Department of Obstetrics and Gynecology, Faculty of Veterinary Medicine, Ankara University, 06110 Ankara, Turkey.
- 3Department of Obstetrics and Gynecology, Faculty of Veterinary Medicine, Kirikkale University, 71450 Kirikkale, Turkey.
- KMID: 2420930
- DOI: http://doi.org/10.4142/jvs.2018.19.5.620
Abstract
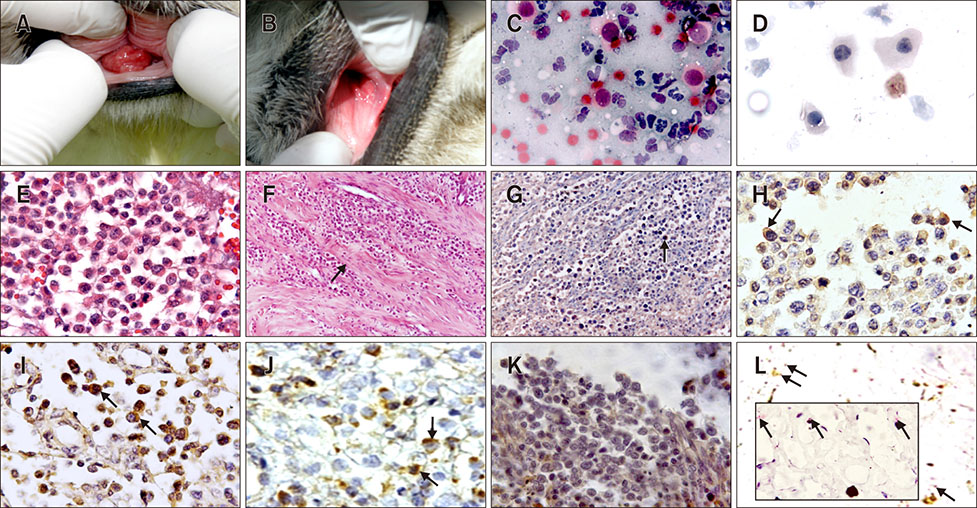
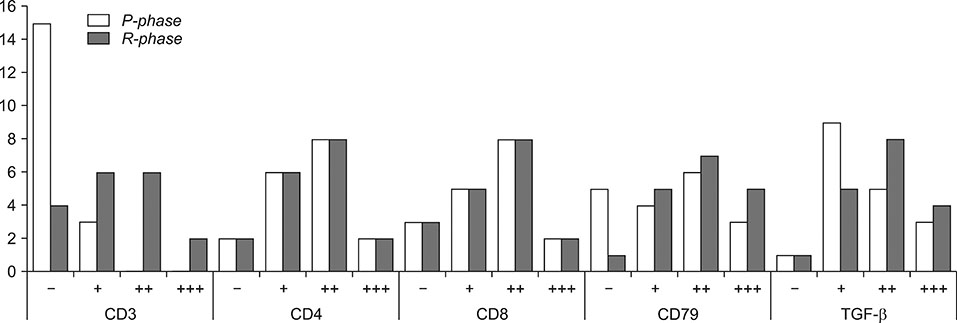
- Canine transmissible venereal tumor (CTVT) is a tumor that commonly occurs in genital and extragenital sites of both genders. Long interspersed nuclear elements (LINE-1) retrotransposon has a pivotal role in allogenic transfection among uncontrolled dog populations. This study aimed to perform pathomorphological, immunohistochemical, and in situ polymerase chain reaction (PCR) evaluation of CTVT (n = 18) in transfected dogs during chemotherapy. Immunohistochemically, tumor phases were investigated by using specific markers (CD3, CD4, CD8, CD79, and transforming growth factor beta [TGF-β]), and investigated an amplified specific sequence of TVT LINE-1 retrotransposon by in situ PCR. Polyhedral-shaped neoplastic cells that had large, round, hypo/hyperchromatic nuclei and eosinophilic cytoplasm were detected. All marker results were positive, especially in the early weeks of recovery. CD4 and TGF-β markers were conspicuously positive at the initial stage. In situ PCR LINE-1 sequence was initially positive in only four cases. It is believed that the CD and TGF-β markers provide phase identification at tumor initiation and during chemotherapy. It is thought that presence of T and B lymphocytes, which have roles in cellular and humoral immunity, is needed so that regression of the tumor is possible.
Keyword
MeSH Terms
Figure
Reference
-
1. Alcigir ME, Atalay Vural S. Evaluation of liver and heart lesions induced by experimental fowl adenovirus-4 infection in broilers and virus detection by immunohistochemistry, immunofluorescence and in situ PCR. Revue Méd Vét. 2013; 164:348–357.2. Chamary JV, Hurst LD. The price of silent mutations. Sci Am. 2009; 300:46–53.
Article3. Choi YK, Kim CJ. Sequence analysis of canine LINE-1 elements and p53 gene in canine transmissible venereal tumor. J Vet Sci. 2002; 3:285–292.
Article4. Fonseca LS, Mota LSLS, Colodel MM, Ferreira I, Brandão CVS, Rocha NS. Spontaneous canine transmissible venereal tumor: association between different phenotypes and the insertion LINE-1/c-myc. Rev Colom Cienc Pecua. 2012; 25:402–408.5. Goldschmidt MH, Hendrick MJ. Tumors of the skin and soft tissues. In : Meuten DJ, editor. Tumors of Domestic Animals. 4th ed. Ames: Iowa State Press;2002. p. 115–117.6. Gonzalez CM, Griffey SM, Naydan DK, Flores E, Cepeda R, Cattaneo G, Madewell BR. Canine transmissible venereal tumour: a morphological and immunohistochemical study of 11 tumours in growth phase and during regression after chemotherapy. J Comp Pathol. 2000; 122:241–248.
Article7. Liao KW, Lin ZY, Pao HN, Kam SY, Wang FI, Chu RM. Identification of canine transmissible venereal tumor cells using in situ polymerase chain reaction and the stable sequence of the long interspersed nuclear element. J Vet Diagn Invest. 2003; 15:399–406.
Article8. McEntee K, Nielsen SW. Tumors of the female genital tract. Bull World Health Organ. 1976; 53:217–226.9. Montoya-Durango DE, Liu Y, Teneng I, Kalbfleisch T, Lacy ME, Steffen MC, Ramos KS. Epigenetic control of mammalian LINE-1 retrotransposon by retinoblastoma proteins. Mutat Res. 2009; 665:20–28.
Article10. Mozos E, Méndez A, Gómez-Villamandos JC, Martín De Las Mulas J, Pérez J. Immunohistochemical characterization of canine transmissible venereal tumor. Vet Pathol. 1996; 33:257–263.
Article11. Mukaratirwa S, Gruys E. Canine transmissible venereal tumour: cytogenetic origin, immunophenotype, and immunobiology. A review. Vet Q. 2003; 25:101–111.
Article12. Murphy SK, Jirtle RL. Imprinting evolution and the price of silence. Bioessays. 2003; 25:577–588.
Article13. Nak D, Nak Y, Cangul IT, Tuna B. A Clinico-pathological study on the effect of vincristine on transmissible venereal tumour in dogs. J Vet Med A Physiol Pathol Clin Med. 2005; 52:366–370.
Article14. Pérez J, Day MJ, Mozos E. Immunohistochemical study of the local inflammatory infiltrate in spontaneous canine transmissible venereal tumour at different stages of growth. Vet Immunol Immunopathol. 1998; 64:133–147.
Article15. Richardson RC. Canine transmissible veneral tumor. Compend Contin Educ Prac Vet. 1981; 3:951–956.16. Rogers KS. Transmissible venereal tumor. Compend Contin Educ Pract Vet. 1997; 19:1036–1045.17. Scarpelli KC, Valladão ML, Metze K. Predictive factors for the regression of canine transmissible venereal tumor during vincristine therapy. Vet J. 2010; 183:362–363.
Article18. Schlafer DH, Miller RB. Female genital system. In : Maxie MG, editor. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. New York: Elsevier Saunders;2007. p. 429–564.19. Smit AF. Interspersed repeats and other mementos of transposable elements in mammalian genomes. Curr Opin Genet Dev. 1999; 9:657–663.
Article20. Trompieri-Silveira AC, Gerardi D, Mouro JV, Costa MT, Alessi AC. Immunohistochemical expression of B and T-lymphocytes and TGF-β in experimentally transplanted canine venereal tumor. Cienc Rural. 2009; 39:1148–1154.
Article21. Wilkins AS. The enemy within: an epigenetic role of retrotransposons in cancer initiation. Bioessays. 2010; 32:856–865.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TP53 Polymorphisms allow for genetic sub-grouping of the canine transmissible venereal tumor
- Sequence analysis of canine LINE-1 elements and p53 gene in canine transmissible venereal tumor
- Molecular detection of Toxoplasma gondii in ticks and their respective host dogs
- Spiral CT for the Detection of Metastatic Tumor of the Liver: Relative Value of Arterial, Portal Venous and Delayed Phase Scanning
- The Mechanisms of Resistance to TNF in TNF-Sensitive Cancer Cells Transfected with TNF-alpha Gene Using Retroviral Vector