Tuberc Respir Dis.
2018 Jan;81(1):29-41. 10.4046/trd.2017.0120.
Tumor Immunology and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea.
- 2Department of Thoracic Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA. scott.antonia@moffitt.org
- KMID: 2420589
- DOI: http://doi.org/10.4046/trd.2017.0120
Abstract
- Lung cancer is one of the most commonly diagnosed cancers and the leading cause of cancer-related deaths worldwide. Although progress in the treatment of advanced non-small cell lung cancer (NSCLC) has been made over the past decade, the 5-year survival rate in patients with lung cancer remains only 10%-20%. Obviously, new therapeutic options are required for patients with advanced NSCLC and unmet medical needs. Cancer immunotherapy is an evolving treatment modality that uses a patient's own immune systems to fight cancer. Theoretically, cancer immunotherapy can result in long-term cancer remission and may not cause the same side effects as chemotherapy and radiation. Immuno-oncology has become an important focus of basic research as well as clinical trials for the treatment of NSCLC. Immune checkpoint inhibitors are the most promising approach for cancer immunotherapy and they have become the standard of care for patients with advanced NSCLC. This review summarizes basic tumor immunology and the relevant clinical data on immunotherapeutic approaches, especially immune checkpoint inhibitors in NSCLC.
MeSH Terms
Figure
Cited by 1 articles
-
Current Status of Immunotherapy for Lung Cancer and Future Perspectives
Ho Cheol Kim, Chang-Min Choi
Tuberc Respir Dis. 2020;83(1):14-19. doi: 10.4046/trd.2019.0039.
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.
Article2. Korea Central Cancer Registry. Annual report of cancer statistics in Korea in 2013 [Internet]. Goyang: National Cancer Information Center;2016. cited 2016 Jun 20. Available from: http://www.cancer.go.kr/.3. Korean Statistical Information Service. Statistics Korea [Internet]. Daejeon: Statistics Korea;2016. cited 2016 Jun 20. Available from: http://kosis.kr/.4. Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013; 382:709–719. PMID: 23972814.
Article5. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in oncology: non-small cell lung cancer. Version 9. 2017 [Internet]. Fort Washington: National Comprehensive Cancer Network;2017. cited 2017 Sep 28. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.6. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015; 5:2892–2911. PMID: 26609494.7. Park JY, Jang SH. Epidemiology of lung cancer in Korea: recent trends. Tuberc Respir Dis (Seoul). 2016; 79:58–69. PMID: 27064578.
Article8. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015; 385:977–1010. PMID: 25467588.
Article9. Shepherd FA, Douillard JY, Blumenschein GR Jr. Immunotherapy for non-small cell lung cancer: novel approaches to improve patient outcome. J Thorac Oncol. 2011; 6:1763–1773. PMID: 21876456.
Article10. Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol. 2012; 13:e301–e310. PMID: 22748269.
Article11. Reck M. What future opportunities may immuno-oncology provide for improving the treatment of patients with lung cancer? Ann Oncol. 2012; 23(Suppl 8):viii28–viii34. PMID: 22918925.
Article12. Brahmer JR. Harnessing the immune system for the treatment of non-small-cell lung cancer. J Clin Oncol. 2013; 31:1021–1028. PMID: 23401435.
Article13. Massarelli E, Papadimitrakopoulou V, Welsh J, Tang C, Tsao AS. Immunotherapy in lung cancer. Transl Lung Cancer Res. 2014; 3:53–63. PMID: 25806281.14. Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res. 2015; 21:976–984. PMID: 25733707.
Article15. Carbone DP, Gandara DR, Antonia SJ, Zielinski C, Paz-Ares L. Non-small-cell lung cancer: role of the immune system and potential for immunotherapy. J Thorac Oncol. 2015; 10:974–984. PMID: 26134219.
Article16. Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. 9th ed. Philadelphia: Saunders/Elsevier;2018.17. Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother. 2012; 35:299–308. PMID: 22495387.18. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011; 29:235–271. PMID: 21219185.
Article19. Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012; 23(Suppl 8):viii6–viii9. PMID: 22918931.
Article20. Eggermont AM. Can immuno-oncology offer a truly pan-tumour approach to therapy? Ann Oncol. 2012; 23(Suppl 8):viii53–viii57. PMID: 22918930.
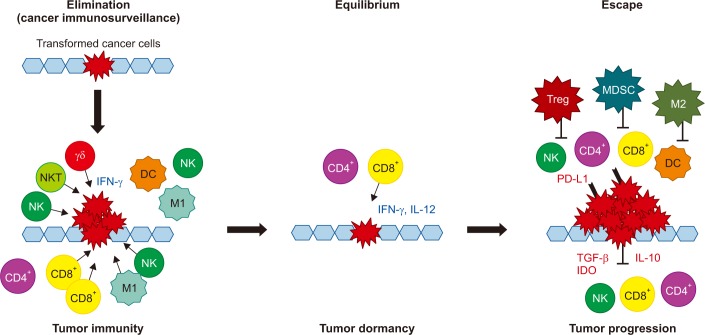
Article21. Burnet M. Cancer: a biological approach. I. The processes of control. Br Med J. 1957; 1:779–786. PMID: 13404306.22. Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001; 410:1107–1111. PMID: 11323675.23. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002; 3:991–998. PMID: 12407406.
Article24. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011; 331:1565–1570. PMID: 21436444.
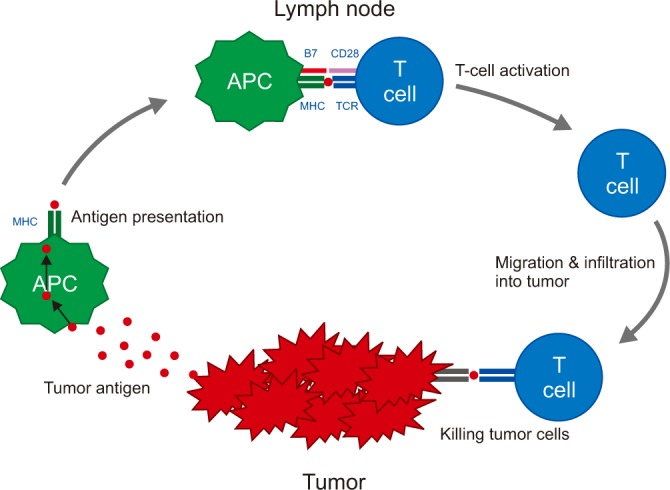
Article25. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013; 39:1–10. PMID: 23890059.
Article26. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011; 480:480–489. PMID: 22193102.
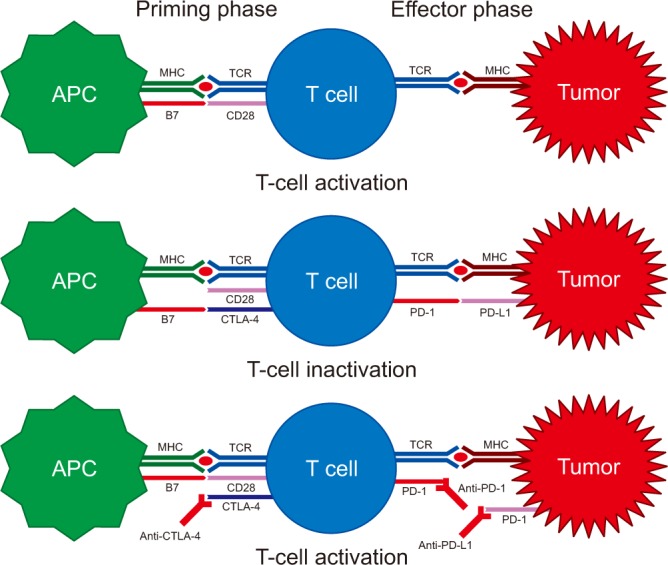
Article27. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–264. PMID: 22437870.
Article28. Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 2014; 33:4623–4631. PMID: 24141774.
Article29. Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, Macri L, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009; 87:365–371. PMID: 19161739.
Article30. Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006; 94:275–280. PMID: 16421594.
Article31. Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH Jr, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006; 107:2866–2872. PMID: 17099880.
Article32. Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014; 11:24–37. PMID: 24247168.
Article33. Vansteenkiste J, Zielinski M, Linder A, Dahabreh J, Gonzalez EE, Malinowski W, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol. 2013; 31:2396–2403. PMID: 23715567.
Article34. Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016; 17:822–835. PMID: 27132212.
Article35. Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014; 15:59–68. PMID: 24331154.
Article36. Wu YL, Park K, Soo RA, Sun Y, Tyroller K, Wages D, et al. INSPIRE: a phase III study of the BLP25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer. BMC Cancer. 2011; 11:430. PMID: 21982342.
Article37. Quoix E, Lena H, Losonczy G, Forget F, Chouaid C, Papai Z, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016; 17:212–223. PMID: 26727163.
Article38. Rodriguez PC, Popa X, Martinez O, Mendoza S, Santiesteban E, Crespo T, et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2016; 22:3782–3790. PMID: 26927662.
Article39. Sasada T, Komatsu N, Suekane S, Yamada A, Noguchi M, Itoh K. Overcoming the hurdles of randomised clinical trials of therapeutic cancer vaccines. Eur J Cancer. 2010; 46:1514–1519. PMID: 20413296.
Article40. Mellstedt H, Vansteenkiste J, Thatcher N. Vaccines for the treatment of non-small cell lung cancer: investigational approaches and clinical experience. Lung Cancer. 2011; 73:11–17. PMID: 21474197.
Article41. Cuppens K, Vansteenkiste J. Vaccination therapy for non-small-cell lung cancer. Curr Opin Oncol. 2014; 26:165–170. PMID: 24441502.
Article42. Makkouk A, Weiner GJ. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res. 2015; 75:5–10. PMID: 25524899.
Article43. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–135. PMID: 26028407.
Article44. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–1639. PMID: 26412456.
Article45. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017; 376:2415–2426. PMID: 28636851.
Article46. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015; 372:2018–2028. PMID: 25891174.
Article47. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–1550. PMID: 26712084.
Article48. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–1833. PMID: 27718847.
Article49. Brahmer JR, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD-L1 tumor proportion score (TPS) ≥50% enrolled in KEYNOTE-024. J Clin Oncol. 2017; 35(15 Suppl):9000.
Article50. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016; 387:1837–1846. PMID: 26970723.
Article51. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017; 389:255–265. PMID: 27979383.
Article52. Garassino M, Vansteenkiste J, Kim JH, Lena H, Mazieres J, Powderly J, et al. Durvalumab in ≥3rd-line locally advanced or metastatic, EGFR/ALK wild-type NSCLC: results from the phase 2 ATLANTIC study. J Thorac Oncol. 2017; 12(Suppl):S10–S11.53. Peters S, Gettinger S, Johnson ML, Janne PA, Garassino MC, Christoph D, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017; 35:2781–2789. PMID: 28609226.54. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017; 377:1919–1929. PMID: 28885881.
Article55. Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012; 30:2046–2054. PMID: 22547592.
Article56. Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017; 35:3449–3457. PMID: 28854067.
Article57. Champiat S, Ileana E, Giaccone G, Besse B, Mountzios G, Eggermont A, et al. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol. 2014; 9:144–153. PMID: 24419410.
Article58. Antonia SJ, Larkin J, Ascierto PA. Immuno-oncology combinations: a review of clinical experience and future prospects. Clin Cancer Res. 2014; 20:6258–6268. PMID: 25341541.
Article59. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016; 17:1497–1508. PMID: 27745820.
Article60. Borghaei H, Langer CJ, Gadgeel S, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Updated results from KEYNOTE-021 cohort G: a randomized, phase 2 study of pemetrexed and carboplatin (PC) with or without pembrolizumab (pembro) as first-line therapy for advanced nonsquamous NSCLC. Ann Oncol. 2017; 28(Suppl_5):636–637.
Article61. Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016; 34:2969–2979. PMID: 27354481.
Article62. Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017; 18:31–41. PMID: 27932067.
Article63. Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016; 17:299–308. PMID: 26858122.
Article64. Remon J, Chaput N, Planchard D. Predictive biomarkers for programmed death-1/programmed death ligand immune checkpoint inhibitors in nonsmall cell lung cancer. Curr Opin Oncol. 2016; 28:122–129. PMID: 26756384.
Article65. Gridelli C, Ardizzoni A, Barberis M, Cappuzzo F, Casaluce F, Danesi R, et al. Predictive biomarkers of immunotherapy for non-small cell lung cancer: results from an Experts Panel Meeting of the Italian Association of Thoracic Oncology. Transl Lung Cancer Res. 2017; 6:373–386. PMID: 28713682.
Article66. Chae YK, Pan A, Davis AA, Raparia K, Mohindra NA, Matsangou M, et al. Biomarkers for PD-1/PD-L1 blockade therapy in non-small-cell lung cancer: is PD-L1 expression a good marker for patient selection? Clin Lung Cancer. 2016; 17:350–361. PMID: 27137346.67. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016; 17:e542–e551. PMID: 27924752.
Article68. Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016; 2:1217–1222. PMID: 27310809.69. Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017; 12:208–222. PMID: 27913228.
Article70. Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017; 3:1051–1058. PMID: 28278348.
Article71. Buttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017; 35:3867–3876. PMID: 29053400.72. Scheerens H, Malong A, Bassett K, Boyd Z, Gupta V, Harris J, et al. Current status of companion and complementary diagnostics: strategic considerations for development and launch. Clin Transl Sci. 2017; 10:84–92. PMID: 28121072.
Article73. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013; 499:214–218. PMID: 23770567.
Article74. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015; 348:124–128. PMID: 25765070.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunotherapy for Non-small-cell Lung Cancer: Current Status and Future Obstacles
- Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
- Current status of cancer immunotherapy with immune checkpoint inhibitors
- Perioperative immunotherapy in stage IB-III non-small cell lung cancer: a critical review of its rationale and considerations
- Treatment of advanced urogenital cancers with immune checkpoint inhibitors