Comparison of the Effects of Ezetimibe-Statin Combination Therapy on Major Adverse Cardiovascular Events in Patients with and without Diabetes: A Meta-Analysis
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. edgo@yuhs.ac
- 2Graduate School, Yonsei University College of Medicine, Seoul, Korea.
- 3Institute of Endocrine Research, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Cardiovascular Medicine, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan.
- 5Department of Medicine (Cardiology) and Radiology, University of Virginia Health System, Charlottesville, VA, USA.
- 6Second Department of Medicine, Department of Cardiovascular Medicine, First Faculty of Medicine, Charles University in Prague and General University Hospital in Prague, Prague, Czech Republic.
- 7Vascular Surgery Unit, Department of Surgery, University of Ioannina, Ioannina, Greece.
- 8Musashino Tokusyu Hospital, Tokyo, Japan.
- 9Department of Biostatistics, The Catholic University of Korea, Seoul, Korea.
- 10Division of Cardiology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 11Cardiovascular Research Institute and Cardiovascular Genome Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2420490
- DOI: http://doi.org/10.3803/EnM.2018.33.2.219
Abstract
- BACKGROUND
Ezetimibe-statin combination therapy has been found to reduce low density lipoprotein cholesterol levels and the risk of major adverse cardiovascular events (MACEs) in large trials. We sought to examine the differential effect of ezetimibe on MACEs when added to statins according to the presence of diabetes.
METHODS
Randomized clinical trials with a sample size of at least 50 participants and at least 24 weeks of follow-up that compared ezetimibe-statin combination therapy with a statin- or placebo-controlled arm and reported at least one MACE, stratified by diabetes status, were included in the meta-analysis and meta-regression.
RESULTS
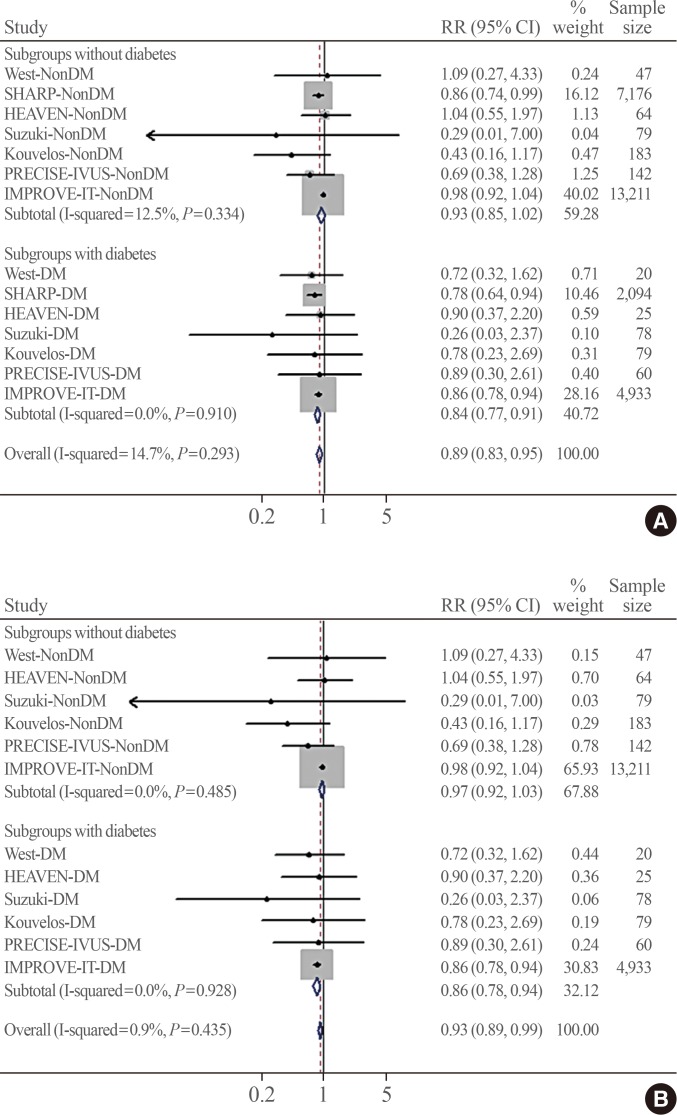
A total of seven trials with 28,191 enrolled patients (mean age, 63.6 years; 75.1% men; 7,298 with diabetes [25.9%]; mean follow-up, 5 years) were analysed. MACEs stratified by diabetes were obtained from the published data (two trials) or through direct contact (five trials). No significant heterogeneity was observed among studies (I 2=14.7%, P=0.293). Ezetimibe was associated with a greater reduction of MACE risk in subjects with diabetes than in those without diabetes (pooled relative risk, 0.84 vs. 0.93; P heterogeneity=0.012). In the meta-regression analysis, the presence of diabetes was associated with a greater reduction of MACE risk when ezetimibe was added to statins (β=0.87, P=0.038).
CONCLUSION
Ezetimibe-statin combination therapy was associated with greater cardiovascular benefits in patients with diabetes than in those without diabetes. Our findings suggest that ezetimibe-statin combination therapy might be a useful strategy in patients with diabetes at a residual risk of MACEs.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
2021 Clinical Practice Guidelines for Diabetes Mellitus in Korea
Kyu Yeon Hur, Min Kyong Moon, Jong Suk Park, Soo-Kyung Kim, Seung-Hwan Lee, Jae-Seung Yun, Jong Ha Baek, Junghyun Noh, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Ye Seul Yang, Jang Won Son, Jong Han Choi, Kee Ho Song, Nam Hoon Kim, Sang Yong Kim, Jin Wha Kim, Sang Youl Rhee, You-Bin Lee, Sang-Man Jin, Jae Hyeon Kim, Chong Hwa Kim, Dae Jung Kim, SungWan Chun, Eun-Jung Rhee, Hyun Min Kim, Hyun Jung Kim, Donghyun Jee, Jae Hyun Kim, Won Seok Choi, Eun-Young Lee, Kun-Ho Yoon, Seung-Hyun Ko
Diabetes Metab J. 2021;45(4):461-481. doi: 10.4093/dmj.2021.0156.Triennial Report of
Endocrinology and Metabolism , 2015 to 2017
Eun-Jung Rhee, Hey Yeon Jang, Won-Young Lee
Endocrinol Metab. 2018;33(2):195-201. doi: 10.3803/EnM.2018.33.2.195.Does Rosuvastatin/Ezetimibe Combination Therapy Offer Potential Benefits for Glucose Metabolism beyond Lipid-Lowering Efficacy in T2DM?
Il Rae Park, Jun Sung Moon
Diabetes Metab J. 2024;48(3):387-389. doi: 10.4093/dmj.2024.0168.2023 Clinical Practice Guidelines for Diabetes Management in Korea: Full Version Recommendation of the Korean Diabetes Association
Jun Sung Moon, Shinae Kang, Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, Yoon Ju Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae Jin Kim, Hyun Min Kim, Jung Hae Ko, Nam Hoon Kim, Chong Hwa Kim, Jeeyun Ahn, Tae Jung Oh, Soo-Kyung Kim, Jaehyun Kim, Eugene Han, Sang-Man Jin, Jaehyun Bae, Eonju Jeon, Ji Min Kim, Seon Mee Kang, Jung Hwan Park, Jae-Seung Yun, Bong-Soo Cha, Min Kyong Moon, Byung-Wan Lee
Diabetes Metab J. 2024;48(4):546-708. doi: 10.4093/dmj.2024.0249.
Reference
-
1. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005; 366:1267–1278. PMID: 16214597.2. Cholesterol Treatment Trialists' (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376:1670–1681. PMID: 21067804.3. Gerstein HC, Yusuf S. Dysglycaemia and risk of cardiovascular disease. Lancet. 1996; 347:949–950. PMID: 8598762.
Article4. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63(25 Pt B):2889–2934. PMID: 24239923.5. ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362:1563–1574. PMID: 20228404.
Article6. Park SW. Intestinal and hepatic niemann-pick c1-like 1. Diabetes Metab J. 2013; 37:240–248. PMID: 23991401.
Article7. Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002; 106:1943–1948. PMID: 12370217.
Article8. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387–2397. PMID: 26039521.
Article9. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011; 377:2181–2192. PMID: 21663949.10. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339:b2535. PMID: 19622551.
Article11. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. PMID: 22008217.
Article12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.
Article13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–612. PMID: 19414839.
Article14. Sedgwick P. Meta-analyses: heterogeneity and subgroup analysis. BMJ. 2013; 346:f4040.
Article15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634. PMID: 9310563.
Article16. West AM, Anderson JD, Meyer CH, Epstein FH, Wang H, Hagspiel KD, et al. The effect of ezetimibe on peripheral arterial atherosclerosis depends upon statin use at baseline. Atherosclerosis. 2011; 218:156–162. PMID: 21570685.
Article17. Kovarnik T, Mintz GS, Skalicka H, Kral A, Horak J, Skulec R, et al. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: HEAVEN study. Circ J. 2012; 76:176–183. PMID: 22076422.18. Kouvelos GN, Arnaoutoglou EM, Matsagkas MI, Kostara C, Gartzonika C, Bairaktari ET, et al. Effects of rosuvastatin with or without ezetimibe on clinical outcomes in patients undergoing elective vascular surgery: results of a pilot study. J Cardiovasc Pharmacol Ther. 2013; 18:5–12. PMID: 22573476.19. Suzuki H, Watanabe Y, Kumagai H, Shuto H. Comparative efficacy and adverse effects of the addition of ezetimibe to statin versus statin titration in chronic kidney disease patients. Ther Adv Cardiovasc Dis. 2013; 7:306–315. PMID: 24280596.
Article20. Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol. 2015; 66:495–507. PMID: 26227186.21. Savarese G, De Ferrari GM, Rosano GM, Perrone-Filardi P. Safety and efficacy of ezetimibe: a meta-analysis. Int J Cardiol. 2015; 201:247–252. PMID: 26301648.
Article22. Fei Y, Guyatt GH, Alexander PE, El Dib R, Siemieniuk RA, Vandvik PO, et al. Addition of Ezetimibe to statins for patients at high cardiovascular risk: systematic review of patient-important outcomes. J Eval Clin Pract. 2018; 24:222–231. PMID: 28090731.
Article23. Battaggia A, Donzelli A, Font M, Molteni D, Galvano A. Clinical efficacy and safety of Ezetimibe on major cardiovascular endpoints: systematic review and meta-analysis of randomized controlled trials. PLoS One. 2015; 10:e0124587. PMID: 25915909.
Article24. Thomopoulos C, Skalis G, Michalopoulou H, Tsioufis C, Makris T. Effect of low-density lipoprotein cholesterol lowering by ezetimibe/simvastatin on outcome incidence: overview, meta-analyses, and meta-regression analyses of randomized trials. Clin Cardiol. 2015; 38:763–769. PMID: 26282344.
Article25. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016; 37:2999–3058. PMID: 27567407.
Article26. Committee for the Korean Guidelines for the Management of Dyslipidemia. 2015 Korean guidelines for the management of dyslipidemia: executive summary (English translation). Korean Circ J. 2016; 46:275–306. PMID: 27275165.27. Nayor M, Vasan RS. Recent update to the US cholesterol treatment guidelines: a comparison with international guidelines. Circulation. 2016; 133:1795–1806. PMID: 27143546.28. Ravid Z, Bendayan M, Delvin E, Sane AT, Elchebly M, Lafond J, et al. Modulation of intestinal cholesterol absorption by high glucose levels: impact on cholesterol transporters, regulatory enzymes, and transcription factors. Am J Physiol Gastrointest Liver Physiol. 2008; 295:G873–G885. PMID: 18772361.
Article29. Lally SE, Owens D, Tomkin GH. Sitosterol and cholesterol in chylomicrons of type 2 diabetic and non-diabetic subjects: the relationship with ATP binding cassette proteins G5 and G8 and Niemann-Pick C1-like 1 mRNA. Diabetologia. 2007; 50:217–219. PMID: 17102949.
Article30. Vaverkova H, Farnier M, Averna M, Missault L, Viigimaa M, Dong Q, et al. Lipid-altering efficacy of ezetimibe/simvastatin 10/20 mg compared to rosuvastatin 10 mg in high-risk patients with and without type 2 diabetes mellitus inadequately controlled despite prior statin monotherapy. Cardiovasc Ther. 2012; 30:61–74. PMID: 20626402.
Article31. Leiter LA, Betteridge DJ, Farnier M, Guyton JR, Lin J, Shah A, et al. Lipid-altering efficacy and safety profile of combination therapy with ezetimibe/statin vs. statin monotherapy in patients with and without diabetes: an analysis of pooled data from 27 clinical trials. Diabetes Obes Metab. 2011; 13:615–628. PMID: 21332628.
Article32. Koh KK, Oh PC, Sakuma I, Kim EY, Lee Y, Hayashi T, et al. Vascular and metabolic effects of ezetimibe combined with simvastatin in patients with hypercholesterolemia. Int J Cardiol. 2015; 199:126–131. PMID: 26188833.
Article33. Tie C, Gao K, Zhang N, Zhang S, Shen J, Xie X, et al. Ezetimibe attenuates atherosclerosis associated with lipid reduction and inflammation inhibition. PLoS One. 2015; 10:e0142430. PMID: 26555472.
Article34. Patel SB. Ezetimibe: a novel cholesterol-lowering agent that highlights novel physiologic pathways. Curr Cardiol Rep. 2004; 6:439–442. PMID: 15485605.
Article35. Qin L, Yang YB, Yang YX, Gong YZ, Li XL, Li GY, et al. Inhibition of smooth muscle cell proliferation by ezetimibe via the cyclin D1-MAPK pathway. J Pharmacol Sci. 2014; 125:283–291. PMID: 25048018.
Article36. Hussein O, Minasian L, Itzkovich Y, Shestatski K, Solomon L, Zidan J. Ezetimibe's effect on platelet aggregation and LDL tendency to peroxidation in hypercholesterolaemia as monotherapy or in addition to simvastatin. Br J Clin Pharmacol. 2008; 65:637–645. PMID: 18241285.
Article37. Peto R, Emberson J, Landray M, Baigent C, Collins R, Clare R, et al. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008; 359:1357–1366. PMID: 18765432.
Article38. Drazen JM, D'Agostino RB, Ware JH, Morrissey S, Curfman GD. Ezetimibe and cancer: an uncertain association. N Engl J Med. 2008; 359:1398–1399. PMID: 18765434.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacological Strategies beyond Statins: Ezetimibe and PCSK9 Inhibitors
- Expanding the therapeutic landscape: ezetimibe as non-statin therapy for dyslipidemia
- Comparison of the Effects of Highintensity Statin Therapy with Moderate-Intensity Statin and Ezetimibe Combination Therapy on Major Adverse Cardiovascular Events in Patients with Acute Myocardial Infarction: a Nationwide Cohort Study
- Combination of Statin and Ezetimibe versus Statin Monotherapy on Cardiovascular Disease and Type 2 Diabetes Incidence among Adults with Impaired Fasting Glucose: a PropensityMatched Nationwide Cohort Study
- Combination pharmacotherapy in lipid management