Endocrinol Metab.
2018 Jun;33(2):164-174. 10.3803/EnM.2018.33.2.164.
Genetic Polymorphism Predisposing to Differentiated Thyroid Cancer: A Review of Major Findings of the Genome-Wide Association Studies
- Affiliations
-
- 1Department of Radiation Molecular Epidemiology, Atomic Bomb Disease Institute, Nagasaki University, Nagasaki, Japan. saenko@nagasaki-u.ac.jp
- 2Department of Global Health, Medicine and Welfare, Atomic Bomb Disease Institute, Nagasaki University, Nagasaki, Japan.
- KMID: 2420483
- DOI: http://doi.org/10.3803/EnM.2018.33.2.164
Abstract
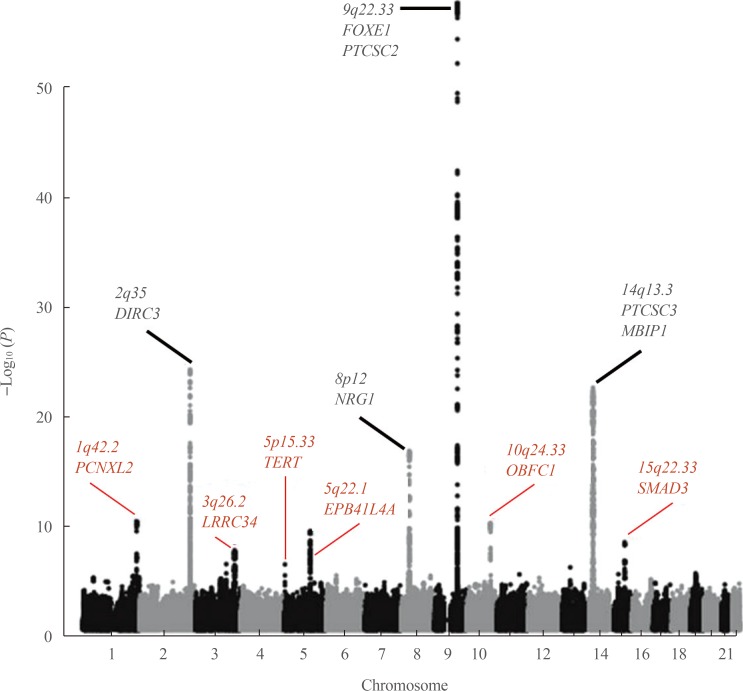
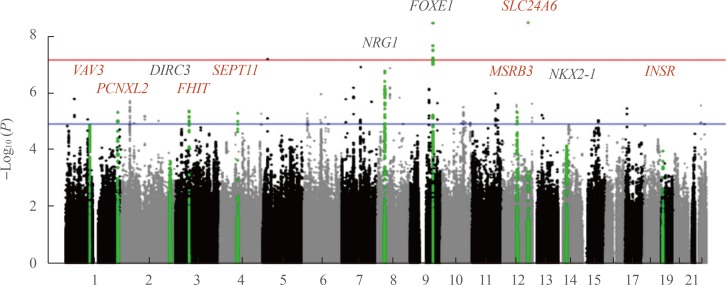
- Thyroid cancer has one of the highest hereditary component among human malignancies as seen in medical epidemiology investigations, suggesting the potential meaningfulness of genetic studies. Here we review researches into genetic variations that influence the chance of developing non-familial differentiated thyroid cancer (DTC), focusing on the major findings of the genome-wide association studies (GWASs) of common single-nucleotide polymorphisms (SNPs). To date, eight GWAS have been performed, and the association of a number of SNPs have been reproduced in dozens of replication investigations across different ethnicities, including Korea and Japan. Despite the cumulative effect of the strongest SNPs demonstrates gradual increase in the risk for cancer and their association signals are statistically quite significant, the overall prediction ability for DTC appears to be very limited. Thus, genotyping of common SNPs only would be insufficient for evidence-based counseling in clinical setting at present. Further studies to include less significant and rare SNPs, non-SNP genetic information, gene-gene interactions, ethnicity, non-genetic and environmental factors, and development of more advanced computational algorithms are warranted to approach to personalized disease risk prediction and prognostication.
Keyword
MeSH Terms
Figure
Reference
-
1. Statistics Korea. Cancer incident cases and incidence rates by site (24 items) and sex [Internet]. Daejeon: Statistics Korea;c2014. cited 2018 May 3. Available from: http://kosis.kr/eng/statisticsList/statisticsList_01List.jsp?vwcd=MT_ETITLE&parentId=D#SubCont.2. Center for Cancer Control and Information Services, National Cancer Center. Projected cancer statistics, 2017 [Internet]. Tokyo: National Cancer Center;2017. updated 2017 Oct 27. cited 2018 May 3. Available from: https://ganjoho.jp/en/public/statistics/short_pred.html.3. Saika K, Matsuda T, Sobue T. Incidence rate of thyroid cancer by histological type in Japan. Jpn J Clin Oncol. 2014; 44:1131–1132. PMID: 25359987.
Article4. Sueta A, Ito H, Kawase T, Hirose K, Hosono S, Yatabe Y, et al. A genetic risk predictor for breast cancer using a combination of low-penetrance polymorphisms in a Japanese population. Breast Cancer Res Treat. 2012; 132:711–721. PMID: 22160591.
Article5. Meigs JB, Shrader P, Sullivan LM, McAteer JB, Fox CS, Dupuis J, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med. 2008; 359:2208–2219. PMID: 19020323.
Article6. National Center for Biotechnology Information. NCBI dbSNP Build 151 [Internet]. Bethesda: NCBI;2018. updated 2018 Mar 22. cited 2018 May 3. Available from: https://www.ncbi.nlm.nih.gov/projects/SNP/snp_summary.cgi?view+summary=view+summary&build_id=151.7. Carvajal-Carmona LG. Challenges in the identification and use of rare disease-associated predisposition variants. Curr Opin Genet Dev. 2010; 20:277–281. PMID: 20564784.
Article8. Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int J Cancer. 2002; 99:260–266. PMID: 11979442.
Article9. Kerber RA, O'Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005; 103:1906–1915. PMID: 15779016.
Article10. Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994; 86:1600–1608. PMID: 7932824.
Article11. Stephens LA, Powell NG, Grubb J, Jeremiah SJ, Bethel JA, Demidchik EP, et al. Investigation of loss of heterozygosity and SNP frequencies in the RET gene in papillary thyroid carcinoma. Thyroid. 2005; 15:100–104. PMID: 15753666.
Article12. Rogounovitch TI, Saenko VA, Ashizawa K, Sedliarou IA, Namba H, Abrosimov AY, et al. TP53 codon 72 polymorphism in radiation-associated human papillary thyroid cancer. Oncol Rep. 2006; 15:949–956. PMID: 16525684.
Article13. Akulevich NM, Saenko VA, Rogounovitch TI, Drozd VM, Lushnikov EF, Ivanov VK, et al. Polymorphisms of DNA damage response genes in radiation-related and sporadic papillary thyroid carcinoma. Endocr Relat Cancer. 2009; 16:491–503. PMID: 19286843.
Article14. Damiola F, Byrnes G, Moissonnier M, Pertesi M, Deltour I, Fillon A, et al. Contribution of ATM and FOXE1 (TTF2) to risk of papillary thyroid carcinoma in Belarusian children exposed to radiation. Int J Cancer. 2014; 134:1659–1668. PMID: 24105688.15. Lonjou C, Damiola F, Moissonnier M, Durand G, Malakhova I, Masyakin V, et al. Investigation of DNA repair-related SNPs underlying susceptibility to papillary thyroid carcinoma reveals MGMT as a novel candidate gene in Belarusian children exposed to radiation. BMC Cancer. 2017; 17:328. PMID: 28499365.
Article16. Landa I, Ruiz-Llorente S, Montero-Conde C, Inglada-Perez L, Schiavi F, Leskela S, et al. The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. PLoS Genet. 2009; 5:e1000637. PMID: 19730683.
Article17. Dudbridge F, Gusnanto A. Estimation of significance thresholds for genomewide association scans. Genet Epidemiol. 2008; 32:227–234. PMID: 18300295.
Article18. Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet. 2009; 41:460–464. PMID: 19198613.
Article19. Takahashi M, Saenko VA, Rogounovitch TI, Kawaguchi T, Drozd VM, Takigawa-Imamura H, et al. The FOXE1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Hum Mol Genet. 2010; 19:2516–2523. PMID: 20350937.
Article20. Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Masson G, He H, et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat Genet. 2012; 44:319–322. PMID: 22267200.
Article21. Kohler A, Chen B, Gemignani F, Elisei R, Romei C, Figlioli G, et al. Genome-wide association study on differentiated thyroid cancer. J Clin Endocrinol Metab. 2013; 98:E1674–E1681. PMID: 23894154.22. Mancikova V, Cruz R, Inglada-Perez L, Fernandez-Rozadilla C, Landa I, Cameselle-Teijeiro J, et al. Thyroid cancer GWAS identifies 10q26.12 and 6q14.1 as novel susceptibility loci and reveals genetic heterogeneity among populations. Int J Cancer. 2015; 137:1870–1878. PMID: 25855579.
Article23. Figlioli G, Chen B, Elisei R, Romei C, Campo C, Cipollini M, et al. Novel genetic variants in differentiated thyroid cancer and assessment of the cumulative risk. Sci Rep. 2015; 5:8922. PMID: 25753578.
Article24. Gudmundsson J, Thorleifsson G, Sigurdsson JK, Stefansdottir L, Jonasson JG, Gudjonsson SA, et al. A genome-wide association study yields five novel thyroid cancer risk loci. Nat Commun. 2017; 8:14517. PMID: 28195142.
Article25. Bychkov A, Saenko V, Nakashima M, Mitsutake N, Rogounovitch T, Nikitski A, et al. Patterns of FOXE1 expression in papillary thyroid carcinoma by immunohistochemistry. Thyroid. 2013; 23:817–828. PMID: 23327367.
Article26. Matsuse M, Takahashi M, Mitsutake N, Nishihara E, Hirokawa M, Kawaguchi T, et al. The FOXE1 and NKX2-1 loci are associated with susceptibility to papillary thyroid carcinoma in the Japanese population. J Med Genet. 2011; 48:645–648. PMID: 21730105.
Article27. Rogounovitch TI, Bychkov A, Takahashi M, Mitsutake N, Nakashima M, Nikitski AV, et al. The common genetic variant rs944289 on chromosome 14q13.3 associates with risk of both malignant and benign thyroid tumors in the Japanese population. Thyroid. 2015; 25:333–340. PMID: 25562676.
Article28. Nikitski AV, Rogounovitch TI, Bychkov A, Takahashi M, Yoshiura KI, Mitsutake N, et al. Genotype analyses in the Japanese and Belarusian populations reveal independent effects of rs965513 and rs1867277 but do not support the role of FOXE1 polyalanine tract length in conferring risk for papillary thyroid carcinoma. Thyroid. 2017; 27:224–235. PMID: 27824288.
Article29. Wang YL, Feng SH, Guo SC, Wei WJ, Li DS, Wang Y, et al. Confirmation of papillary thyroid cancer susceptibility loci identified by genome-wide association studies of chromosomes 14q13, 9q22, 2q35 and 8p12 in a Chinese population. J Med Genet. 2013; 50:689–695. PMID: 23847140.
Article30. Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013; 3:520–533. PMID: 23365119.
Article31. He H, Li W, Liyanarachchi S, Jendrzejewski J, Srinivas M, Davuluri RV, et al. Genetic predisposition to papillary thyroid carcinoma: involvement of FOXE1, TSHR, and a novel lincRNA gene, PTCSC2. J Clin Endocrinol Metab. 2015; 100:E164–E172. PMID: 25303483.
Article32. Nikitski A, Saenko V, Shimamura M, Nakashima M, Matsuse M, Suzuki K, et al. Targeted Foxe1 overexpression in mouse thyroid causes the development of multinodular goiter but does not promote carcinogenesis. Endocrinology. 2016; 157:2182–2195. PMID: 26982637.
Article33. Jendrzejewski J, He H, Radomska HS, Li W, Tomsic J, Liyanarachchi S, et al. The polymorphism rs944289 predis poses to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc Natl Acad Sci U S A. 2012; 109:8646–8651. PMID: 22586128.34. Luhrig S, Siamishi I, Tesmer-Wolf M, Zechner U, Engel W, Nolte J. Lrrc34, a novel nucleolar protein, interacts with npm1 and ncl and has an impact on pluripotent stem cells. Stem Cells Dev. 2014; 23:2862–2874. PMID: 24991885.35. Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013; 20:603–610. PMID: 23766237.
Article36. Matsuse M, Yabuta T, Saenko V, Hirokawa M, Nishihara E, Suzuki K, et al. TERT promoter mutations and Ki-67 labeling index as a prognostic marker of papillary thyroid carcinomas: combination of two independent factors. Sci Rep. 2017; 7:41752. PMID: 28150740.
Article37. Ishigaki K, Namba H, Nakashima M, Nakayama T, Mitsutake N, Hayashi T, et al. Aberrant localization of beta-catenin correlates with overexpression of its target gene in human papillary thyroid cancer. J Clin Endocrinol Metab. 2002; 87:3433–3440. PMID: 12107263.38. Law MH, Bishop DT, Lee JE, Brossard M, Martin NG, Moses EK, et al. Genome-wide meta-analysis identifies five new susceptibility loci for cutaneous malignant melanoma. Nat Genet. 2015; 47:987–995. PMID: 26237428.39. Kimura ET, Kopp P, Zbaeren J, Asmis LM, Ruchti C, Maciel RM, et al. Expression of transforming growth factor beta1, beta2, and beta3 in multinodular goiters and differentiated thyroid carcinomas: a comparative study. Thyroid. 1999; 9:119–125. PMID: 10090310.40. Son HY, Hwangbo Y, Yoo SK, Im SW, Yang SD, Kwak SJ, et al. Genome-wide association and expression quantitative trait loci studies identify multiple susceptibility loci for thyroid cancer. Nat Commun. 2017; 8:15966. PMID: 28703219.
Article42. Riesco-Eizaguirre G, Santisteban P. New insights in thyroid follicular cell biology and its impact in thyroid cancer therapy. Endocr Relat Cancer. 2007; 14:957–977. PMID: 18045949.
Article43. Hanai N, Nagata K, Kawajiri A, Shiromizu T, Saitoh N, Hasegawa Y, et al. Biochemical and cell biological characterization of a mammalian septin, Sept11. FEBS Lett. 2004; 568:83–88. PMID: 15196925.
Article44. Joannes A, Bonnomet A, Bindels S, Polette M, Gilles C, Burlet H, et al. Fhit regulates invasion of lung tumor cells. Oncogene. 2010; 29:1203–1213. PMID: 19935706.
Article45. Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, et al. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996; 84:587–597. PMID: 8598045.
Article46. Priya TP, Kapoor VK, Krishnani N, Agrawal V, Agarwal S. Fragile histidine triad (FHIT) gene and its association with p53 protein expression in the progression of gall bladder cancer. Cancer Invest. 2009; 27:764–773. PMID: 19452299.
Article47. Zou M, Shi Y, Farid NR, al-Sedairy ST, Paterson MC. FHIT gene abnormalities in both benign and malignant thyroid tumours. Eur J Cancer. 1999; 35:467–472. PMID: 10448301.
Article48. Yin DT, Wang L, Sun J, Yin F, Yan Q, Shen RL, et al. Homozygous deletion but not mutation of exons 5 and 8 of the fragile histidine triad (FHIT) gene is associated with features of differentiated thyroid carcinoma. Ann Clin Lab Sci. 2010; 40:267–272. PMID: 20689140.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Short History of the Genome-Wide Association Study: Where We Were and Where We Are Going
- The Search for Genetic Variants and Epigenetics Related to Asthma
- Sulwon Lecture 2009: The Search for Genetic Risk Factors of Type 2 Diabetes Mellitus
- Asthma and epigenetics
- Genome-Wide Association Study of Hepatitis in Korean Populations