Transl Clin Pharmacol.
2018 Sep;26(3):134-140. 10.12793/tcp.2018.26.3.134.
Development of a UPLC-MS/MS method for the therapeutic monitoring of L-asparaginase
- Affiliations
-
- 1College of Pharmacy, Research Institute of Pharmaceutical Sciences, Kyungpook National University, Daegu 41566, Korea. kshin@knu.ac.kr
- 2Department of Hematology, Chonnam National University Hwasun Hospital, Hwasun 58128, Korea.
- 3College of Pharmacy and Research Institute of Pharmaceutical Sciences, Seoul National University, Seoul 03080, Korea.
- KMID: 2420296
- DOI: http://doi.org/10.12793/tcp.2018.26.3.134
Abstract
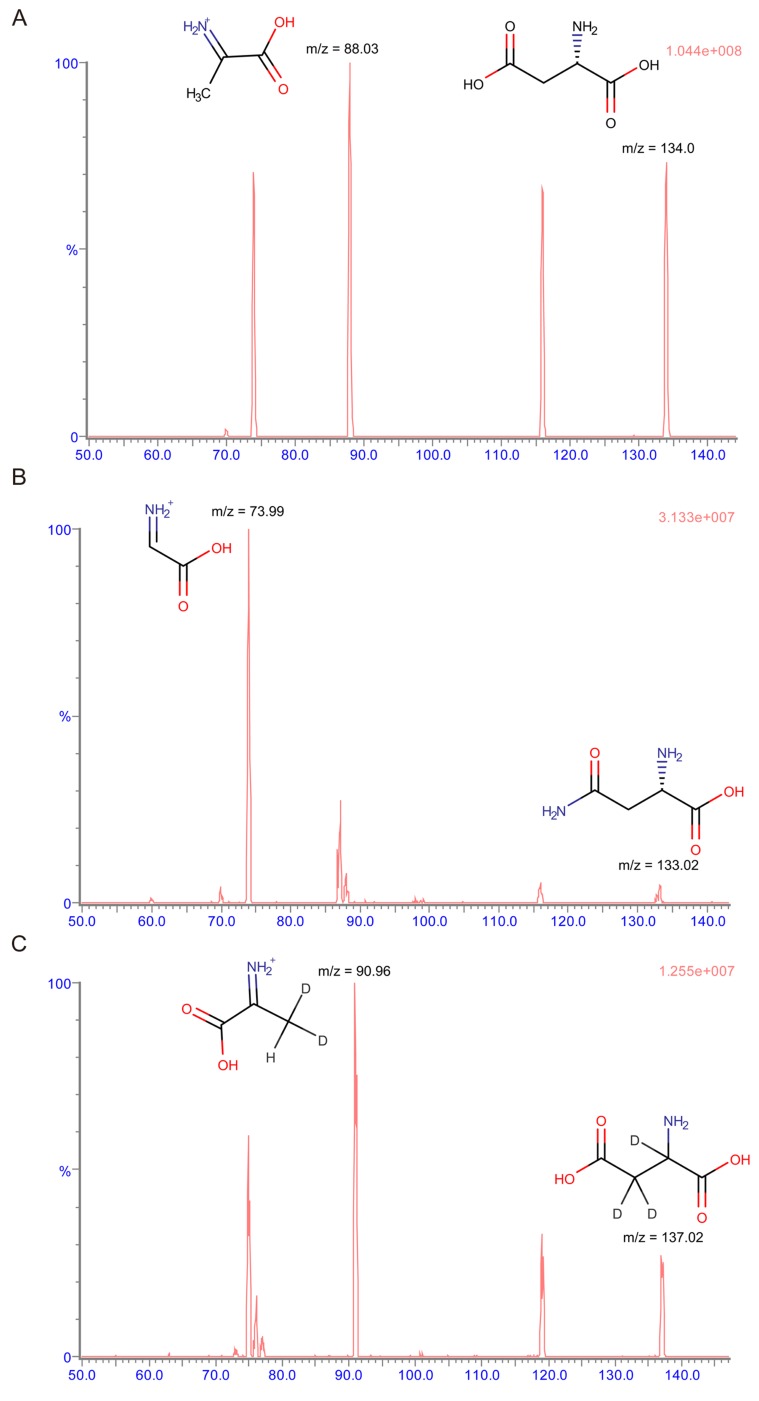
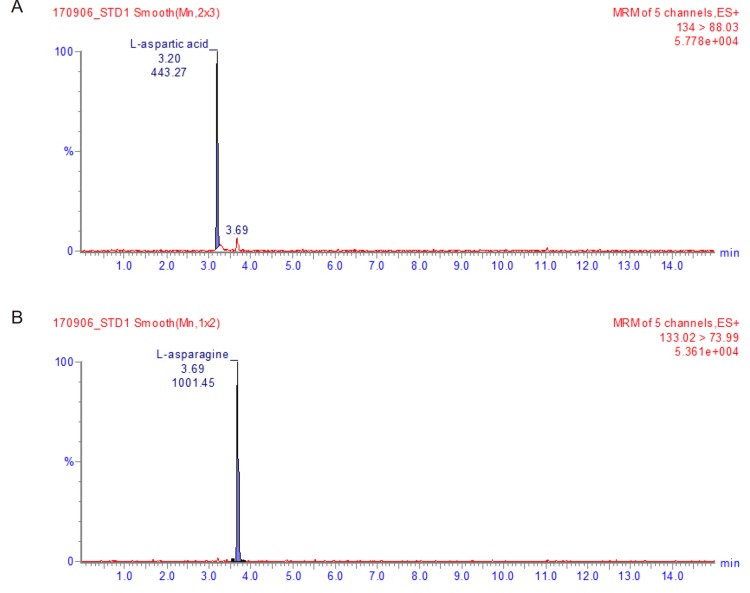
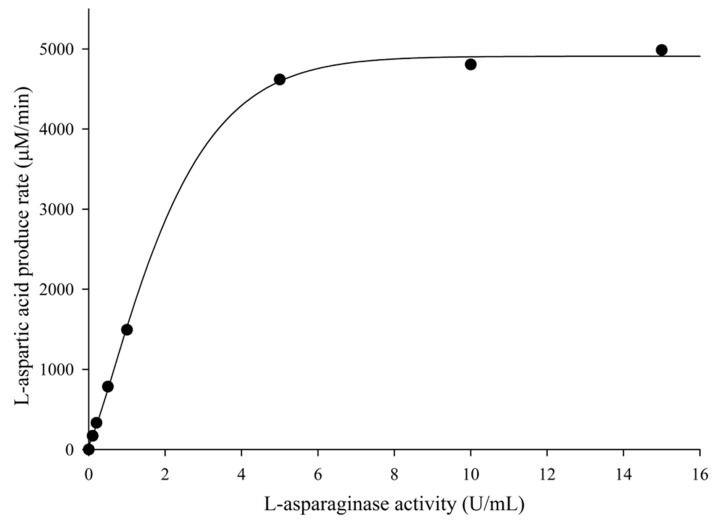
- This study aimed to develop a UPLC-MS/MS method for determining plasma levels of L-aspartic acid and L-asparagine and the activity of L-asparaginase. L-aspartic acid, L-asparagine, and L-aspartic acid-2,3,3-d3 were extracted from human plasma by protein precipitation with sulfosalicylic acid (30%, v/v). The plasma samples were analyzed using an Imtakt Intrada amino acid analysis column with 25 mM ammonium formate and 0.5% formic acid in acetonitrile as the mobile phase with step gradient method at a flow rate of 0.5 mL/min. The injection volume was 5 µL, and the total run time was 15 min. Inter- and intra-batch accuracies (%) ranged from 96.62-106.0% for L-aspartic acid and 89.85-104.8%, for L-asparagine, and the coefficient of variation (CV%) did not exceed 7%. The validation results for L-aspartic acid and L-asparagine satisfied the specified criterion, however, the results for L-asparaginase activity assay showed a borderline validity. This study could be a foundation for further development of therapeutic drug monitoring systems using UPLC-MS/MS.
Keyword
MeSH Terms
Figure
Reference
-
1. Asselin B, Rizzari C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk Lymphoma. 2015; 56:2273–2280. DOI: 10.3109/10428194.2014.1003056. PMID: 25586605.
Article2. Müller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998; 28:97–113. DOI: 10.1016/S1040-8428(98)00015-8. PMID: 9768345.3. Kumar K, Kaur J, Walia S, Pathak T, Aggarwal D. L-asparaginase: an effective agent in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma. 2014; 55:256–262. DOI: 10.3109/10428194.2013.803224. PMID: 23662993.
Article4. Boos J, Werber G, Ahlke E, Schulze-Westhoff P, Nowak-Göttl U, Würthwein G, et al. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur J Cancer. 1996; 32A:1544–1544. DOI: 10.1016/0959-8049(96)00131-1. PMID: 8911116.
Article5. Douer D, Yampolsky H, Cohen LJ, Watkins K, Levine AM, Periclou AP, et al. Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood. 2007; 109:2744–2750. DOI: 10.1182/blood-2006-07-035006. PMID: 17132721.
Article6. Vieira Pinheiro JP, Ahlke E, Nowak-Göttl U, Hempel G, Müller HJ, Lümkemann K, et al. Pharmacokinetic dose adjustment of Erwinia asparaginase in protocol II of the paediatric ALL/NHL-BFM treatment protocols. Br J Haematol. 1999; 104:313–320. PMID: 10050714.
Article7. Ahlke E, Nowak-Göttl U, Schulze-Westhoff P, Werber G, Börste H, Würthwein G, et al. Dose reduction of asparaginase under pharmacokinetic and pharmacodynamic control during induction therapy in children with acute lymphoblastic leukaemia. Br J Haematol. 1997; 96:675–681. DOI: 10.1046/j.1365-2141.1997.d01-2089.x. PMID: 9074406.
Article8. Rizzari C, Zucchetti M, Conter V, Diomede L, Bruno A, Gavazzi L, et al. L-asparagine depletion and L-asparaginase activity in children with acute lymphoblastic leukemia receiving i.m. or i.v. Erwinia C. or E-coli L-asparaginase as first exposure. Ann Oncol. 2000; 11:189–193. DOI: 10.1023/A:1008368916800. PMID: 10761754.9. Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, et al. L-asparaginase treatment in acute lymphoblastic leukemia. Cancer. 2011; 117:238–249. DOI: 10.1002/cncr.25489. PMID: 20824725.
Article10. Wetzler M, Sanford BL, Kurtzberg J, DeOliveira D, Frankel SR, Powell BL, et al. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511. Blood. 2007; 109:4164–4167. DOI: 10.1182/blood-2006-09-045351. PMID: 17264295.
Article11. Asselin B, Rizzari C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk Lymphoma. 2015; 56:2273–2280. DOI: 10.3109/10428194.2014.1003056. PMID: 25586605.
Article12. Mei L, Ontiveros EP, Griffiths EA, Thompson JE, Wang ES, Wetzler M. Pharmacogenetics predictive of response and toxicity in acute lymphoblastic leukemia therapy. Blood Rev. 2015; 29:243–249. DOI: 10.1016/j.blre.2015.01.001. PMID: 25614322.
Article13. Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999; 13:335–342. PMID: 10086723.
Article14. Pession A, Valsecchi MG, Masera G, Kamps WA, Magyarosy E, Rizzari C, et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. 2005; 23:7161–7167. DOI: 10.1200/JCO.2005.11.411. PMID: 16192600.
Article15. Nath CE, Dallapozza L, Eslick AE, Misra A, Carr D, Earl JW. An isocratic fluorescence HPLC assay for the monitoring of l-asparaginase activity and l-asparagine depletion in children receiving E. colil-asparaginase for the treatment of acute lymphoblastic leukaemia. Biomed Chromatogr. 2009; 23:152–159. DOI: 10.1002/bmc.1096. PMID: 18823071.16. Lanvers C, Vieira Pinheiro JP, Hempel G, Wuerthwein G, Boos J. Analytical validation of a microplate reader-based method for the therapeutic drug monitoring of L-asparaginase in human serum. Anal Biochem. 2002; 309:117–126. PMID: 12381370.
Article17. Kaspar H, Dettmer K, Gronwald W, Oefner PJ. Advances in amino acid analysis. Anal Bioanal Chem. 2009; 393:445–452. DOI: 10.1007/s00216-008-2421-1. PMID: 18843484.
Article18. Thakare R, Chhonker YS, Gautam N, Alamoudi JA, Alnouti Y. Quantitative analysis of endogenous compounds. J Pharm Biomed Anal. 2016; 128:426–437. DOI: 10.1016/j.jpba.2016.06.017. PMID: 27344632.
Article19. Jones BR, Schultz GA, Eckstein JA, Ackermann BL. Surrogate matrix and surrogate analyte approaches for definitive quantitation of endogenous biomolecules. Bioanalysis. 2012; 4:2343–2356. DOI: 10.4155/Bio.12.200. PMID: 23088461.
Article20. Shama N, Bai SW, Chung BC, Jung BH. Quantitative analysis of 17 amino acids in the connective tissue of patients with pelvic organ prolapse using capillary electrophoresis-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008; 865:18–24. DOI: 10.1016/j.jchromb.2008.01.027.
Article21. MFDS. Guideline on Bioanalytical Method Validation. Accessed 6 September 2017. http://nifds.go.kr/_custom/nifds/_common/board/download.jsp?attach_no=18470/.22. Tong WH, Pieters R, Kaspers GJ, te Loo DM, Bierings MB, van den Bos C, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014; 123:2026–2033. DOI: 10.1182/blood-2013-10-534347. PMID: 24449211.
Article23. Schrey D, Borghorst S, Lanvers-Kaminsky C, Hempel G, Gerss J, Möricke A, et al. Therapeutic drug monitoring of asparaginase in the ALL-BFM 2000 protocol between 2000 and 2007. Pediatr Blood Cancer. 2010; 54:952–958. DOI: 10.1002/pbc.22417. PMID: 20108339.
Article24. Lanvers C, Vieira Pinheiro JP, Hempel G, Wuerthwein G, Boos J. Analytical validation of a microplate reader-based method for the therapeutic drug monitoring of L-asparaginase in human serum. Anal Biochem. Anal Biochem; 309:117–126. Pii S0003-2697(02)00232-4. PMID: 12381370.
Article25. Appel IM, Kazemier KM, Boos J, Lanvers C, Huijmans J, Veerman AJ, et al. Pharmacokinetic, pharmacodynamic and intracellular effects of PEG-asparaginase in newly diagnosed childhood acute lymphoblastic leukemia: results from a single agent window study. Leukemia. 2008; 22:1665–1679. DOI: 10.1038/leu.2008.165. PMID: 18580955.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and validation of a UPLC-MS/MS method for the quantification of acetaminophen in human plasma and its application to pharmacokinetic studies
- Development and Validation of a Novel Isotope Dilution-Ultraperformance Liquid ChromatographyTandem Mass Spectrometry Method for Serum C-Peptide
- Clinical Usefulness of Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry Method for Low Serum Testosterone Measurement
- Validated UPLC-MS/MS method for the determination of tadalafil in human plasma and its application to a pharmacokinetic study
- Quantitative Analysis and Enantiomeric Separation of Ephedra Alkaloids in Ma Huang Related Products by HPLC-DAD and UPLC-MS/MS