Obstet Gynecol Sci.
2018 Mar;61(2):227-234. 10.5468/ogs.2018.61.2.227.
Pretreatment neutrophil-to-lymphocyte ratio and its dynamic change during neoadjuvant chemotherapy as poor prognostic factors in advanced ovarian cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Institute of Women's Life Medical Science, Yonsei University College of Medicine, Seoul, Korea. Jungyunlee@yuhs.ac
- KMID: 2420176
- DOI: http://doi.org/10.5468/ogs.2018.61.2.227
Abstract
OBJECTIVE
The purpose of this study was to determine the prognostic implications of the pretreatment neutrophil-to-lymphocyte ratio (NLR) and its dynamic change during chemotherapy in patients with advanced epithelial ovarian cancer undergoing neoadjuvant chemotherapy.
METHODS
We performed a retrospective analysis of 203 patients who underwent neoadjuvant chemotherapy prior to interval debulking surgery for advanced-stage ovarian cancer at Yonsei Cancer Hospital between 2007 and 2015. Pretreatment NLR was evaluated before starting neoadjuvant chemotherapy. Change in NLR was defined as the post-neoadjuvant NLR value divided by the initial value. The correlation of NLR and its dynamic change with chemotherapy response score, response rate, and recurrence was analyzed.
RESULTS
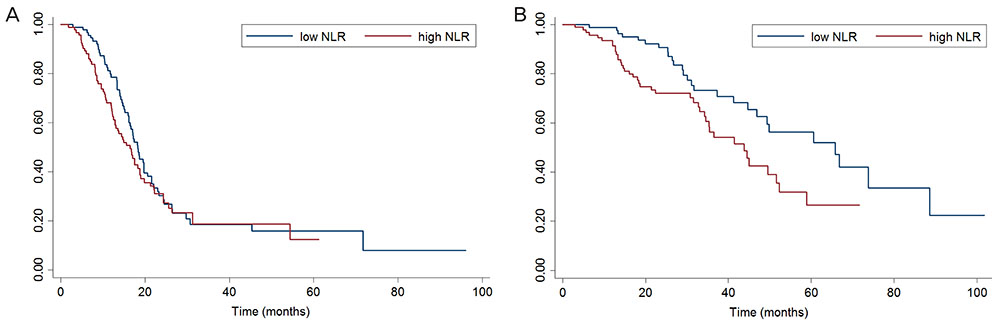
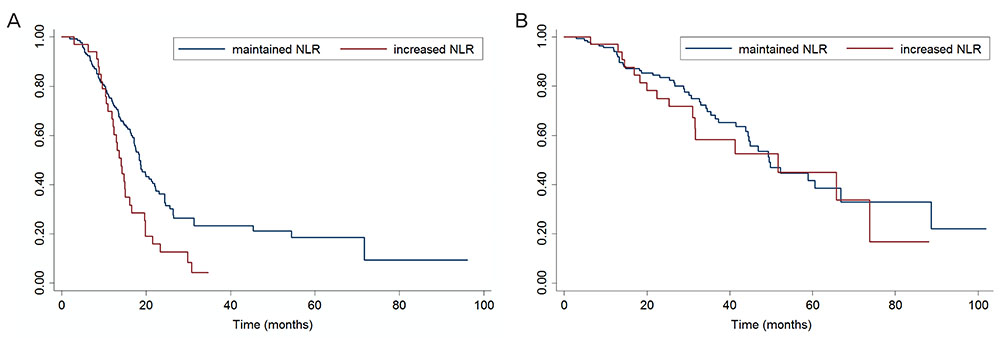
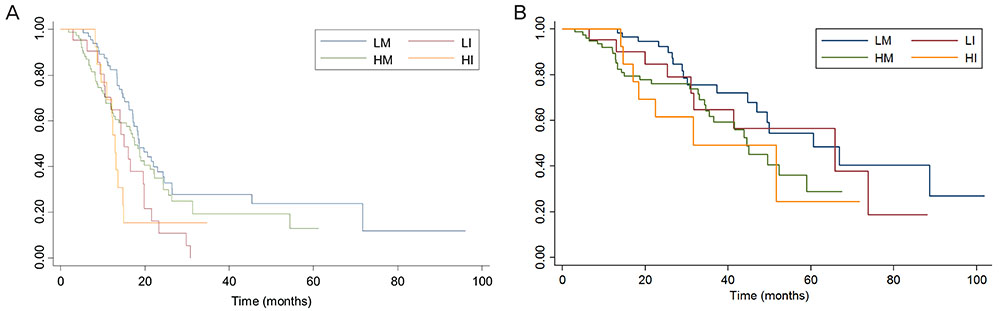
The NLR ranged from 0.64 to 22.8. In univariate analyses, a higher pretreatment NLR (> 3.81) was associated with poor overall survival (OS), but not progression-free survival (PFS). Through multivariate analysis, high pretreatment NLR was shown to be an independent parameter affecting OS, but not necessarily PFS. Changes in NLR during chemotherapy were better predictors of PFS than baseline NLR. Patients with increased NLR during chemotherapy showed significantly poor PFS, and this change was an independent predictor of PFS.
CONCLUSION
Pretreatment NLR and its dynamic change during chemotherapy may be important prognostic factors in patients who undergo neoadjuvant chemotherapy.
MeSH Terms
Figure
Cited by 1 articles
-
Serum lactate dehydrogenase is a possible predictor of platinum resistance in ovarian cancer
Asami Ikeda, Ken Yamaguchi, Hajime Yamakage, Kaoru Abiko, Noriko Satoh-Asahara, Kenji Takakura, Ikuo Konishi
Obstet Gynecol Sci. 2020;63(6):709-718. doi: 10.5468/ogs.20117.
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. Lee JY, Kim EY, Jung KW, Shin A, Chan KK, Aoki D, et al. Trends in gynecologic cancer mortality in East Asian regions. J Gynecol Oncol. 2014; 25:174–182.
Article3. Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999–2010. J Gynecol Oncol. 2013; 24:298–302.
Article4. National Comprehensive Cancer Network (US). Clinical practice guidelines in oncology [Internet]. Washington (PA): National Comprehensive Cancer Network;2016. cited 2016 Dec 13. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.5. Greimel E, Kristensen GB, van der Burg ME, Coronado P, Rustin G, del Rio AS, et al. Quality of life of advanced ovarian cancer patients in the randomized phase III study comparing primary debulking surgery versus neo-adjuvant chemotherapy. Gynecol Oncol. 2013; 131:437–444.
Article6. Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015; 386:249–257.
Article7. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014; 384:1376–1388.
Article8. Cannistra SA. Cancer of the ovary. N Engl J Med. 2004; 351:2519–2529.
Article9. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674.
Article10. Glazer ES, Rashid OM, Pimiento JM, Hodul PJ, Malafa MP. Increased neutrophil-to-lymphocyte ratio after neoadjuvant therapy is associated with worse survival after resection of borderline resectable pancreatic ductal adenocarcinoma. Surgery. 2016; 160:1288–1293.
Article11. Buisan O, Orsola A, Areal J, Font A, Oliveira M, Martinez R, et al. Low pretreatment neutrophil-to-lymphocyte ratio predicts for good outcomes in patients receiving neoadjuvant chemotherapy before radical cystectomy for muscle invasive bladder cancer. Clin Genitourin Cancer. 2017; 15:145–151.e2.
Article12. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005; 91:181–184.
Article13. Chen Y, Chen K, Xiao X, Nie Y, Qu S, Gong C, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016; 16:320.
Article14. Miao Y, Yan Q, Li S, Li B, Feng Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark. 2016; 17:33–40.
Article15. Feng Z, Wen H, Bi R, Ju X, Chen X, Yang W, et al. Preoperative neutrophil-to-lymphocyte ratio as a predictive and prognostic factor for high-grade serous ovarian cancer. PLoS One. 2016; 11:e0156101.
Article16. Yildirim MA, Seckin KD, Togrul C, Baser E, Karsli MF, Gungor T, et al. Roles of neutrophil/lymphocyte and platelet/lymphocyte ratios in the early diagnosis of malignant ovarian masses. Asian Pac J Cancer Prev. 2014; 15:6881–6885.
Article17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.
Article18. Cho KM, Park H, Oh DY, Kim TY, Lee KH, Han SW, et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their dynamic changes during chemotherapy is useful to predict a more accurate prognosis of advanced biliary tract cancer. Oncotarget. 2017; 8:2329–2341.
Article19. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011; 331:1565–1570.
Article20. Güngör N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, et al. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010; 25:149–154.
Article21. Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, et al. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol. 2004; 172:5034–5040.
Article22. Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999; 154:1805–1813.
Article23. Peng W, Li C, Wen TF, Yan LN, Li B, Wang WT, et al. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res. 2014; 192:402–408.
Article24. Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009; 58:15–23.
Article25. Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014; 132:542–550.
Article26. Ashrafganjoei T, Mohamadianamiri M, Farzaneh F, Hosseini MS, Arab M. Investigating preoperative hematologic markers for prediction of ovarian cancer surgical outcome. Asian Pac J Cancer Prev. 2016; 17:1445–1448.
Article27. Thavaramara T, Phaloprakarn C, Tangjitgamol S, Manusirivithaya S. Role of neutrophil to lymphocyte ratio as a prognostic indicator for epithelial ovarian cancer. J Med Assoc Thai. 2011; 94:871–877.28. Wang Y, Liu P, Xu Y, Zhang W, Tong L, Guo Z, et al. Preoperative neutrophil-to-lymphocyte ratio predicts response to first-line platinum-based chemotherapy and prognosis in serous ovarian cancer. Cancer Chemother Pharmacol. 2015; 75:255–262.
Article29. Wang YQ, Jin C, Zheng HM, Zhou K, Shi BB, Zhang Q, et al. A novel prognostic inflammation score predicts outcomes in patients with ovarian cancer. Clin Chim Acta. 2016; 456:163–169.
Article30. Yildirim M, Demir Cendek B, Filiz Avsar A. Differentiation between benign and malignant ovarian masses in the preoperative period using neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios. Mol Clin Oncol. 2015; 3:317–321.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Can pretreatment platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict long-term oncologic outcomes after preoperative chemoradiation followed by surgery for locally advanced rectal cancer?
- The Role of Neutrophil-to-Lymphocyte Ratio in Predicting Pathological Response for Resectable Non–Small Cell Lung Cancer Treated with Neoadjuvant Chemotherapy Combined with PD-1 Checkpoint Inhibitors
- Potential predictors for chemotherapeutic response and prognosis in epithelial ovarian, fallopian tube and primary peritoneal cancer patients treated with platinum-based chemotherapy
- The Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio Are Prognostic Factors in Patients with Locally Advanced Pancreatic Cancer Treated with Chemoradiotherapy
- Clinical Significance of Preoperative Inflammatory Parameters in Gastric Cancer Patients