J Korean Neurosurg Soc.
2018 Sep;61(5):592-599. 10.3340/jkns.2017.0303.005.
Gamma Knife Radiosurgery for Metastatic Brain Tumors with Exophytic Hemorrhage
- Affiliations
-
- 1Department of Neurosurgery, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 2Department of Neurosurgery, Dongtan Sacred Heart Hospital, College of Medicine, Hallym University, Hwaseong, Korea.
- 3Department of Neurosurgery, Dankook University College of Medicine, Cheonan, Korea.
- 4Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ykwon@amc.seoul.kr
- KMID: 2420067
- DOI: http://doi.org/10.3340/jkns.2017.0303.005
Abstract
OBJECTIVE
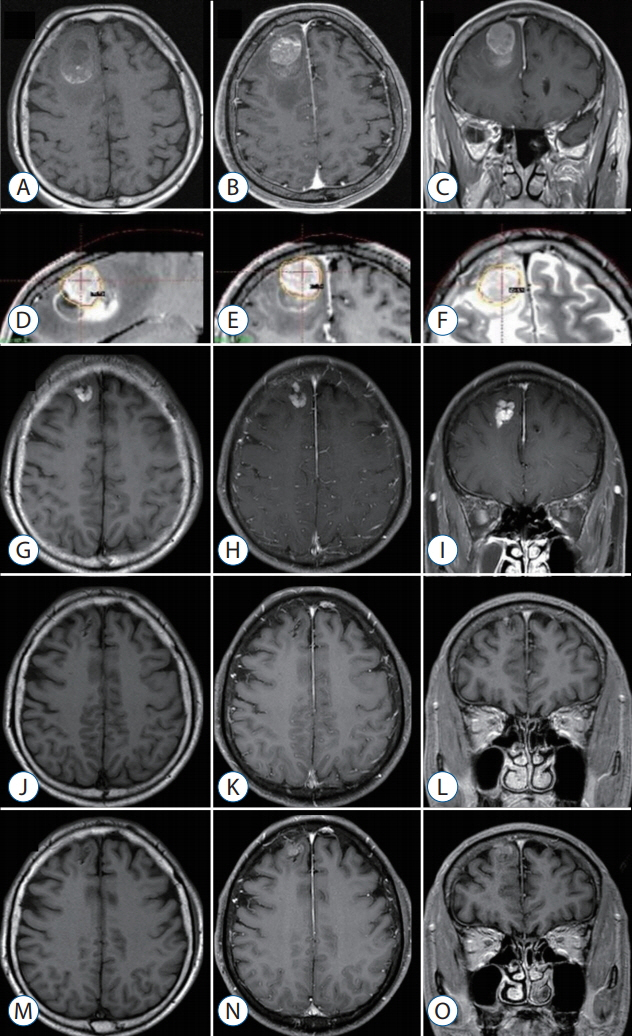
Metastatic brain tumors (MBTs) often present with intracerebral hemorrhage. Although Gamma Knife surgery (GKS) is a valid treatment option for hemorrhagic MBTs, its efficacy is unclear. To achieve oncologic control and reduce radiation toxicity, we used a radiosurgical targeting technique that confines the tumor core within the hematoma when performing GKS in patients with such tumors. We reviewed our experience in this endeavor, focusing on local tumor control and treatment-associated morbidities.
METHODS
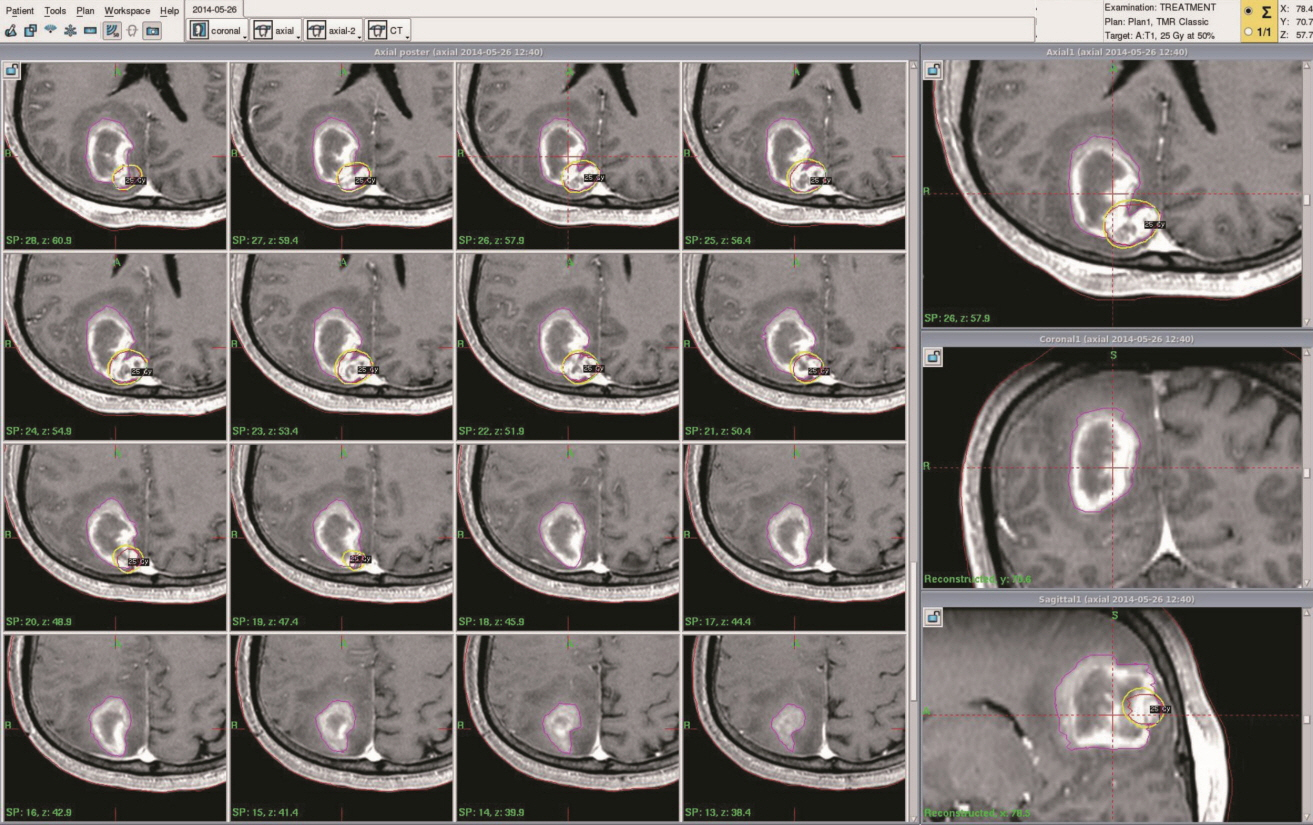
From 2007 to 2014, 13 patients with hemorrhagic MBTs were treated via GKS using our targeting technique. The median marginal dose prescribed was 23 Gy (range, 20-25). GKS was performed approximately 2 weeks after tumor bleeding to allow the patient's condition to stabilize.
RESULTS
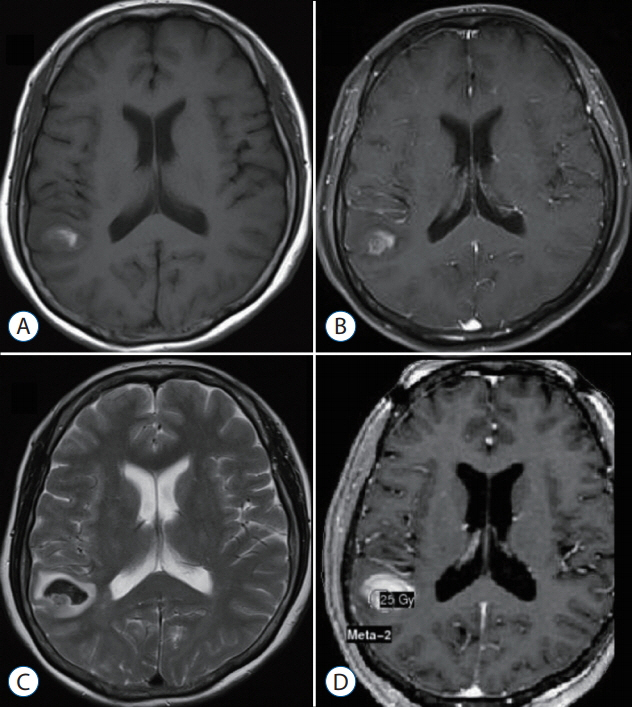
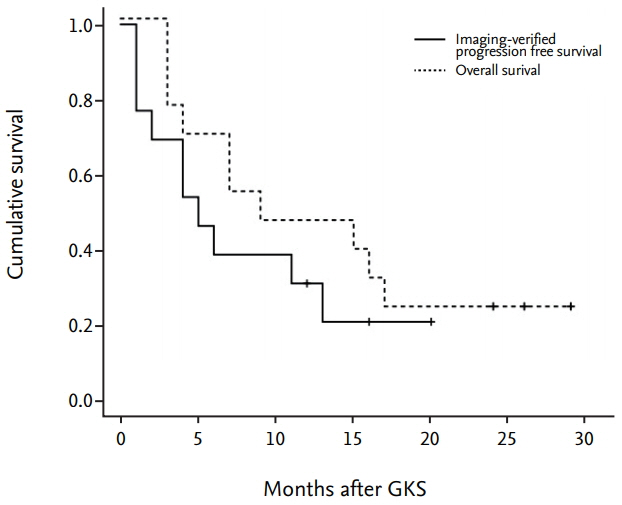
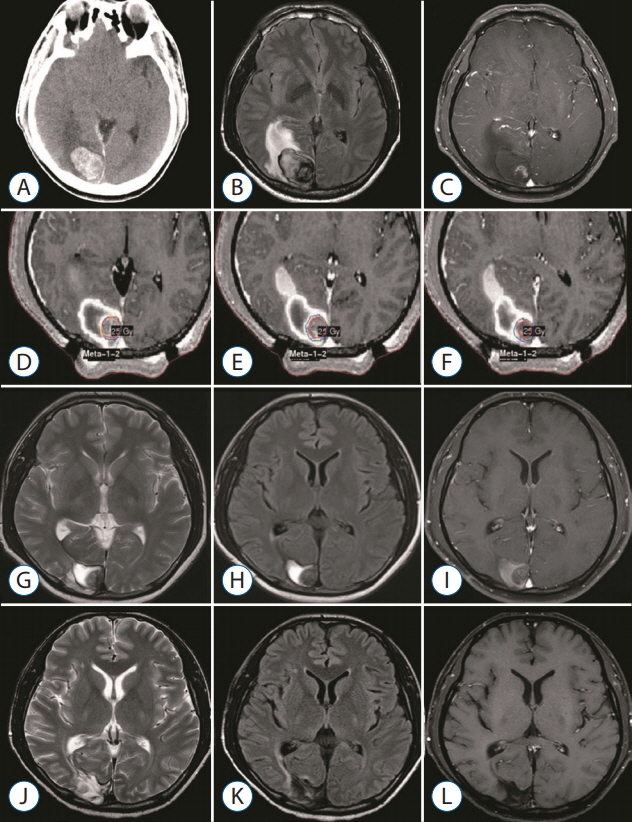
The primary sites of the MBTs included the liver (n=7), lung (n=2), kidney (n=1), and stomach (n=1); in two cases, the primary tumor was a melanoma. The mean tumor volume was 4.00 cm³ (range, 0.74-11.0). The mean overall survival duration after GKS was 12.5 months (range, 3-29), and three patients are still alive at the time of the review. The local tumor control rate was 92% (tumor disappearance 23%, tumor regression 46%, and stable disease 23%). There was one (8%) instance of local recurrence, which occurred 11 months after GKS in the solid portion of the tumor. No GKS-related complications were observed.
CONCLUSION
Our experience shows that GKS performed in conjunction with our targeting technique safely and effectively treats hemorrhagic MBTs. The success of this technique may reflect the presence of scattered metastatic tumor cells in the hematoma that do not proliferate owing to the inadequate microenvironment of the hematoma. We suggest that GKS can be a useful treatment option for patients with hemorrhagic MBTs that are not amenable to surgery.
MeSH Terms
Figure
Reference
-
References
1. Aghi MK, Ogilvy CS, Carter BS. Ch.69 Surgical management of intracerebral hemorrhage. Schmidek & sweet operative neurosurgical techniques: indications, methods, and results. ed 6. Philadelphia: Saunders;2012. p. 825.2. Ajay Niranjan JNJ, Hideyuki Kano , Douglas Kondziolka L, Dade Lunsford , John C, Flickinger . Ch.256 Gamma Knife radiosurgery. Youmans Neurological Surgery. ed 6. Philadelphia: Saunders;2011. p. 2633–2634.3. Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol. 92:275–282. 2009.
Article4. Alexandru D, Bota DA, Linskey ME. Epidemiology of central nervous system metastases. Prog Neurol Surg. 25:13–29. 2012.
Article5. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 363:1665–1672. 2004.
Article6. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 295:2483–2491. 2006.
Article7. Bugyik E, Dezso K, Reiniger L, László V, Tóvári J, Tímár J, et al. Lack of angiogenesis in experimental brain metastases. J Neuropathol Exp Neurol. 70:979–991. 2011.
Article8. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 10:1037–1044. 2009.
Article9. Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 3:53–57. 2002.
Article10. Gregory JA, Murad WAF. Ch.258 Radiosurgery of malignant tumors. Youmans Neurological Surgery. ed 6. Philadelphia: Saunders;2011. p. 2645.11. Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 29:134–141. 2010.12. Kondziolka D, Bernstein M, Resch L, Tator CH, Fleming JR, Vanderlinden RG, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 67:852–857. 1987.
Article13. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 45:427–434. 1999.
Article14. Lee EQ. Nervous system metastases from systemic cancer. Continuum (Minneap Minn). 21(2 Neuro-oncology):415–428. 2015.
Article15. Mut M. Surgical treatment of brain metastasis: a review. Clin Neurol Neurosurg. 114:1–8. 2012.
Article16. Nabors LB, Ammirati M, Bierman PJ, Brem H, Butowski N, Chamberlain MC, et al. Central nervous system cancers. J Natl Compr Canc Netw. 11:1114–1151. 2013.
Article17. Nabors LB, Portnow J, Ammirati M, Brem H, Brown P, Butowski N, et al. Central nervous system cancers, version 2.2014. Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 12:1517–1523. 2014.18. Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 11:203–222. 2014.
Article19. Park ES, Kwon DH, Park JB, Lee DH, Cho YH, Kim JH, et al. Gamma Knife surgery for treating brain metastases arising from hepatocellular carcinomas. J Neurosurg. 121 Suppl:102–109. 2014.
Article20. Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev. (9):CD006121. 2012.
Article21. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 47:291–298. 2000.
Article22. Soon YY, Tham IW, Lim KH, Koh WY, Lu JJ. Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev. (3):CD009454. 2014.
Article23. Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 362:1119–1127. 2010.
Article24. Yaeh A, Nanda T, Jani A, Rozenblat T, Qureshi Y, Saad S, et al. Control of brain metastases from radioresistant tumors treated by stereotactic radiosurgery. J Neurooncol. 124:507–514. 2015.
Article25. Yen CP, Steiner L. Ch.5 Gamma Knife surgery for cerebral vascular malformations and tumors. Schmidek & Sweet operative neurosurgical techniques: indications, methods, and results. ed 6. Philadelphia: Saunders;2011. –514. p. 77–78.26. Yoo H, Jung E, Gwak HS, Shin SH, Lee SH. Surgical outcomes of hemorrhagic metastatic brain tumors. Cancer Res Treat. 43:102–107. 2011.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- How to use Leksell GammaPlan
- Stereotactic radiosurgery for brain metastases
- Gamma Knife Radiosurgery for Single & Multiple brain Metastasis
- Clinical Application of 7.0 T Magnetic Resonance Images in Gamma Knife Radiosurgery for a Patient with Brain Metastases
- Recurrence of pediatric cerebral arteriovenous malformation after obliteration by radiosurgery: a case report