Ann Rehabil Med.
2018 Aug;42(4):502-513. 10.5535/arm.2018.42.4.502.
Effects of Electric Cortical Stimulation (ECS) and Transcranial Direct Current Stimulation (tDCS) on Rats With a Traumatic Brain Injury
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, Presbyterian Medical Center, Jeonju, Korea. foryou6199@naver.com
- 2Medical Device Clinical Trial Center, Presbyterian Medical Center, Jeonju, Korea.
- 3Department of Biological Sciences, Chonbuk National University, Jeonju, Korea.
- 4Department of Family Medicine, Presbyterian Medical Center, Jeonju, Korea.
- KMID: 2420041
- DOI: http://doi.org/10.5535/arm.2018.42.4.502
Abstract
OBJECTIVE
To evaluate the effects of electric cortical stimulation (ECS) and transcranial direct current stimulation (tDCS) on motor and cognitive function recovery and brain plasticity in focal traumatic brain injury (TBI) of rats model.
METHODS
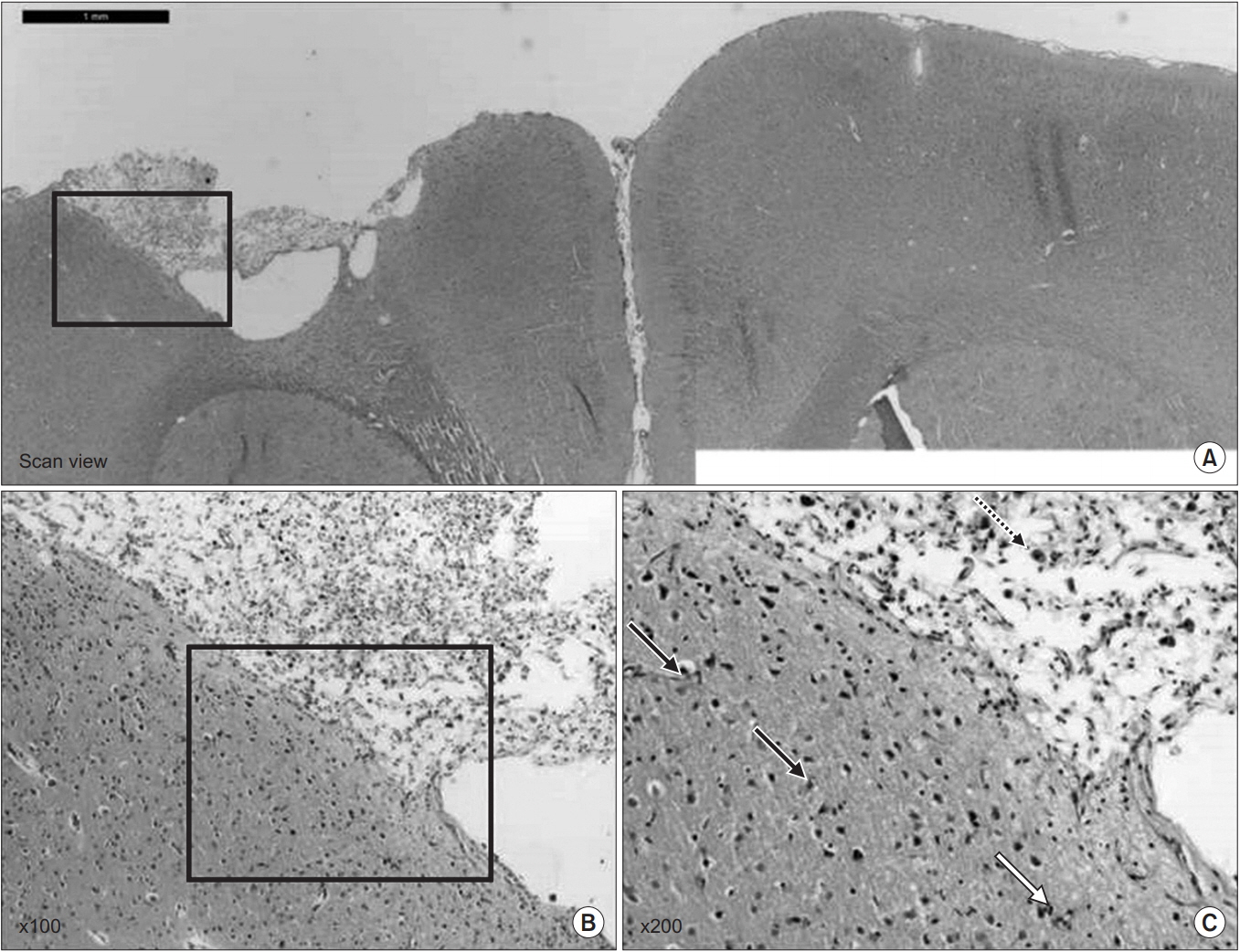
Forty rats were pre-trained to perform a single pellet reaching task (SPRT), rotarod test (RRT), and Y-maze test for 14 days, then a focal TBI was induced by a weight drop model on the motor cortex. All rats were randomly assigned to one of the three groups: anodal ECS (50 Hz and 194 μs) (ECS group), tDCS (0.1 mA, 50 Hz and 200 μs) (tDCS group), and no stimulation as a control group. Four-week stimulation, including rehabilitation, was started 3 days after the operation. SPRT, RRT, and Y-maze were measured from day 1 to day 28 after the TBI was induced. Histopathological and immunohistochemistry staining evaluations were performed at 4 weeks.
RESULTS
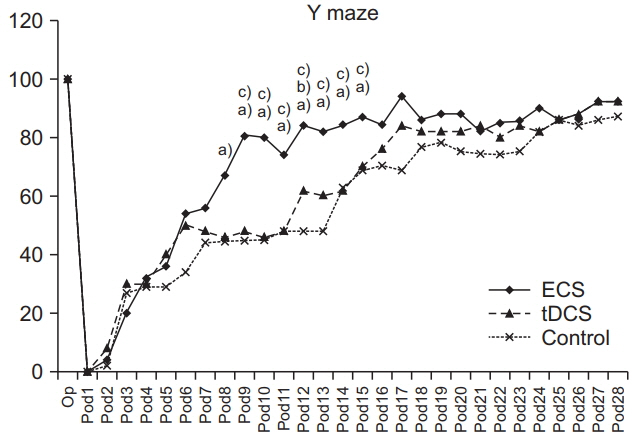
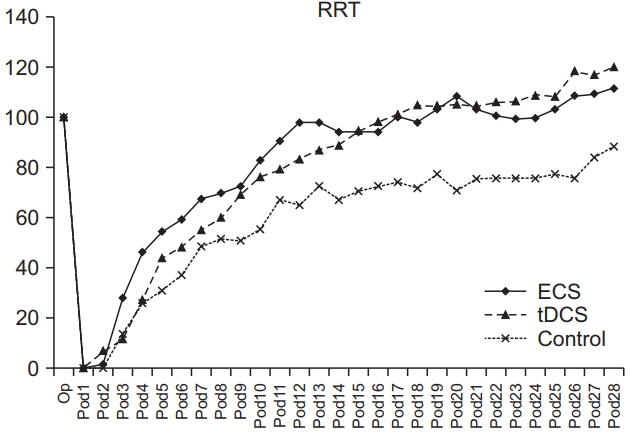
SPRT was improved from day 7 to day 26 in ECS, and from day 8 to day 26 in tDCS compared to the control group (p < 0.05). SPRT of ECS group was significantly improved on days 3, 8, 9, and 17 compared to the tDCS group. Y-maze was improved from day 8 to day 16 in ECS, and on days 6, 12, and 16 in the tDCS group compared to the control group (p < 0.05). Y-maze of the ECS group was significantly improved on day 9 to day 15 compared to the tDCS group. The c-Fos protein expression was better in the ECS group and the tDCS group compared to the control group.
CONCLUSION
Electric stimulation in rats modified with a focal TBI is effective for motor recovery and brain plasticity. ECS induced faster behavioral and cognitive improvements compared to tDCS during the recovery period of rats with a focal TBI.
Keyword
MeSH Terms
Figure
Reference
-
1. Harvey RL, Nudo RJ. Cortical brain stimulation: a potential therapeutic agent for upper limb motor recovery following stroke. Top Stroke Rehabil. 2007; 14:54–67.
Article2. Minhas AS, Woo EJ, Lee SY. Magnetic flux density measurement with balanced steady state free precession pulse sequence for MREIT: a simulation study. Conf Proc IEEE Eng Med Biol Soc. 2009; 2009:2276–8.
Article3. Martin PI, Naeser MA, Theoret H, Tormos JM, Nicholas M, Kurland J, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. 2004; 25:181–91.
Article4. Kim HI, Shin YI, Moon SK, Chung GH, Lee MC, Kim HG. Unipolar and continuous cortical stimulation to enhance motor and language deficit in patients with chronic stroke: report of 2 cases. Surg Neurol. 2008; 69:77–80.
Article5. Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006; 58:464–73.
Article6. Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, et al. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006; 404:232–6.
Article7. Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005; 128(Pt 3):490–9.
Article8. Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003; 15:619–26.
Article9. National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington: National Academies Press;2011.10. Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981; 211:67–77.
Article11. Ducker TB. Experimental injury of the spinal cord. In : Vinken PJ, editor. Handbook of clinical neurology. Amsterdam: Elsevier;1976. p. 9–26.12. Yoon YS, Yu KP, Kim H, Kim HI, Kwak SH, Kim BO. The effect of electric cortical stimulation after focal traumatic brain injury in rats. Ann Rehabil Med. 2012; 36:596–608.
Article13. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. San Diego: Elsevier;2007. p. 30–82.14. Yang CY, Moon SK, Song JH, Kim HS, Han EH, Kim TJ, et al. The effect of continuous epidural electrical stimulation on synapse and neuronal cell in rat with focal ischemia. J Korean Acad Rehabil Med. 2008; 32:375–87.15. Salzman SK, Faden AI. The neurobiology of central nervous system trauma. New York: Oxford University Press;1994. p. 3–12.16. Vergara-Aragon P, Gonzalez CL, Whishaw IQ. A novel skilled-reaching impairment in paw supination on the “good” side of the hemi-Parkinson rat improved with rehabilitation. J Neurosci. 2003; 23:579–86.
Article17. Hunter AJ, Mackay KB, Rogers DC. To what extent have functional studies of ischaemia in animals been useful in the assessment of potential neuroprotective agents? Trends Pharmacol Sci. 1998; 19:59–66.
Article18. Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 1992; 588:132–9.
Article19. Conrad CD, Lupien SJ, Thanasoulis LC, McEwen BS. The effects of type I and type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-maze. Brain Res. 1997; 759:76–83.
Article20. Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995; 26:627–35.21. Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001; 24:1000–19.
Article22. Kreisel SH, Hennerici MG, Bazner H. Pathophysiology of stroke rehabilitation: the natural course of clinical recovery, use-dependent plasticity and rehabilitative outcome. Cerebrovasc Dis. 2007; 23:243–55.
Article23. Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003; 25:789–93.
Article24. Sawaki L, Wu CW, Kaelin-Lang A, Cohen LG. Effects of somatosensory stimulation on use-dependent plasticity in chronic stroke. Stroke. 2006; 37:246–7.
Article25. Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003; 25:801–10.
Article26. Kang C, Yang CY, Kim JH, Moon SK, Lee S, Park SA, et al. The effect of continuous epidural electrical stimulation on neuronal proliferation in cerebral ischemic rats. Ann Rehabil Med. 2013; 37:301–10.
Article27. Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003; 25:780–8.
Article28. Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003; 25:794–800.
Article29. Moon SK, Shin YI, Kim HI, Kim H, Lee JO, Lee MC. Effect of prolonged cortical stimulation differs with size of infarct after sensorimotor cortical lesions in rats. Neurosci Lett. 2009; 460:152–5.
Article30. Kim SJ, Kim BK, Ko YJ, Bang MS, Kim MH, Han TR. Functional and histologic changes after repeated transcranial direct current stimulation in rat stroke model. J Korean Med Sci. 2010; 25:1499–505.
Article31. Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002; 125(Pt 10):2238–47.
Article32. Yoon KJ, Oh BM, Kim DY. Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Res. 2012; 1452:61–72.33. Sim KC, Kim GD, Kim KY, An HJ, Lee JH, Min KO, et al. The effects of tDCS and Montoya stair task on sensorimotor recovery and GFAP expression in MCAo induced stroke rat model. J Int Acad Phys Ther Res. 2011; 2:193–200.
Article34. Yu SH, Park SD, Sim KC. The effect of tDCS on cognition and neurologic recovery of rats with Alzheimer’s disease. J Phys Ther Sci. 2014; 26:247–9.35. Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006; 249:31–8.
Article36. Lacroix L, White I, Feldon J. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behav Brain Res. 2002; 133:69–81.
Article37. Pratt WE, Mizumori SJ. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav Brain Res. 2001; 123:165–83.
Article38. Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005; 64:872–5.
Article39. Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991; 14:421–51.
Article40. Day HE, Kryskow EM, Nyhuis TJ, Herlihy L, Campeau S. Conditioned fear inhibits c-fos mRNA expression in the central extended amygdala. Brain Res. 2008; 1229:137–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcranial Direct Current Stimulation-Psychiatric Application and Its Current Status
- Noninvasive brain stimulation: repetitive transcranial magnetic stimulation and transcranial direct current stimulation
- Therapeutic Application of Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation in Depression
- Safety of Transcranial Direct Current Stimulation in Neurorehabilitation
- Clinical Applications of Transcranial Direct Current Stimulation in Neurological Disorders