Yonsei Med J.
2017 May;58(3):626-630. 10.3349/ymj.2017.58.3.626.
The Need for New Donor Stratification to Predict Graft Survival in Deceased Donor Kidney Transplantation
- Affiliations
-
- 1Department of Surgery, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea.
- 2Transplantation Center, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jbparkmd@gmail.com
- KMID: 2419122
- DOI: http://doi.org/10.3349/ymj.2017.58.3.626
Abstract
- PURPOSE
The aim of this study was to determine whether stratification of deceased donors by the United Network for Organ Sharing (UNOS) criteria negatively impacts graft survival.
MATERIALS AND METHODS
We retrospectively reviewed deceased donor and recipient pretransplant variables of kidney transplantations that occurred between February 1995 and December 2009. We compared clinical outcomes between standard criteria donors (SCDs) and expanded criteria donors (ECDs).
RESULTS
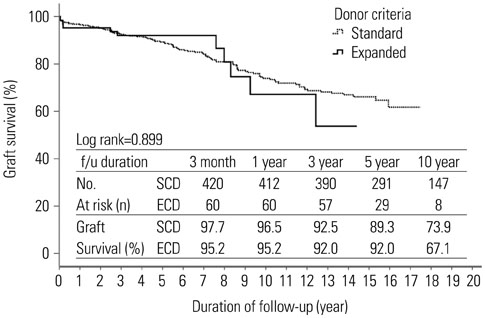
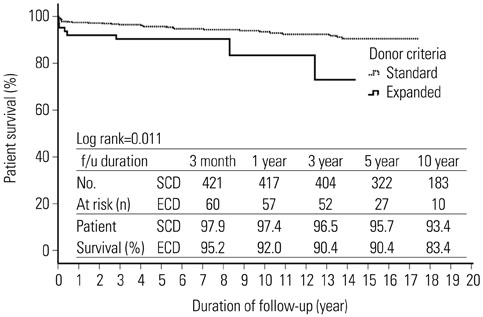
The deceased donors consisted of 369 patients. A total of 494 transplant recipients were enrolled in this study. Mean age was 41.7±11.4 year (range 18-69) and 273 patients (55.4%) were male. Mean duration of follow-up was 8.8±4.9 years. The recipients from ECD kidneys were 63 patients (12.8%). The overall mean cold ischemia time was 5.7±3.2 hours. Estimated glomerular filtration rate at 1, 2, and 3 years after transplantation were significantly lower in ECD transplants (1 year, 62.2±17.6 vs. 51.0±16.4, p<0.001; 2 year, 62.2±17.6 vs. 51.0±16.4, p=0.001; 3 year, 60.9±23.5 vs. 54.1±18.7, p=0.047). In multivariate analysis, donor age (≥40 years) was an independent risk factor for graft failure. In Kaplan-Meier analyses, there was no significant difference in death-censored graft survival (Log rank test, p>0.05), although patient survival was lower in ECDs than SCDs (Log rank test, p=0.011).
CONCLUSION
Our data demonstrate that stratification by the UNOS criteria does not predict graft survival. In order to expand the donor pool, new criteria for standard/expanded donors need to be modified by regional differences.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
*Cadaver
Child
Child, Preschool
Creatinine/blood
Female
Graft Rejection/epidemiology
*Graft Survival
Humans
Infant
Infant, Newborn
Kaplan-Meier Estimate
Kidney/physiopathology
*Kidney Transplantation/adverse effects/mortality/statistics & numerical data
Male
Middle Aged
Postoperative Complications/epidemiology
Retrospective Studies
Risk Factors
Time Factors
*Tissue Donors
Tissue and Organ Procurement/*methods/standards/statistics & numerical data
*Treatment Outcome
United States/epidemiology
Young Adult
Creatinine
Figure
Cited by 3 articles
-
Kidney Transplantation from Expanded Criteria Donor in Korea: It's Time to Have Our Own Criteria Based on Our Experiences
Shin-Seok Yang, Jae Berm Park
J Korean Soc Transplant. 2017;31(1):16-24. doi: 10.4285/jkstn.2017.31.1.16.Clinical Outcomes and Contributors in Contemporary Kidney Transplantation: Single Center Experience
Jae-Sung Ahn, Kyung Sun Park, Jongha Park, Hyun Chul Chung, Hojong Park, Sang Jun Park, Hong Rae Cho, Jong Soo Lee
J Korean Soc Transplant. 2017;31(4):182-192. doi: 10.4285/jkstn.2017.31.4.182.A Preliminary Study to Revise the Marginal Donor Criteria of KONOS in Deceased Donor Kidney Transplantation
Wooseong Huh
J Korean Soc Transplant. 2017;31(2):59-67. doi: 10.4285/jkstn.2017.31.2.59.
Reference
-
1. Noordzij M, Kramer A, Abad Diez JM, Alonso de la Torre R, Arcos Fuster E, Bikbov BT, et al. Renal replacement therapy in Europe: a summary of the 2011 ERA-EDTA registry annual report. Clin Kidney J. 2014; 7:227–238.
Article2. Mathur AK, Ashby VB, Sands RL, Wolfe RA. Geographic variation in end-stage renal disease incidence and access to deceased donor kidney transplantation. Am J Transplant. 2010; 10(4 Pt 2):1069–1080.
Article3. Cusumano AM, Romao JE, Poblete Badal H, Elgueta Miranda S, Gomez R, Cerdas Calderon M, et al. [Latin-American dialysis and kidney transplantation registry: data on the treatment of end-stage renal disease in Latin America]. G Ital Nefrol. 2008; 25:547–553.4. Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002; 74:1281–1286.
Article5. United Network for Organ Sharing. Organ procurement and transplantation network policies. Policy 8: allocation of kidneys. 2016 March 1. Available at:http://optn.transplant.hrsa.gov/governance/policies.6. Kim BS, Joo SH, Ahn HJ, Choi JH, Lee SH, Park HC. Outcomes of expanded-criteria deceased donor kidney transplantation in a single center. Transplant Proc. 2014; 46:1067–1070.
Article7. Lionaki S, Kapsia H, Makropoulos I, Metsini A, Skalioti C, Gakiopoulou H, et al. Kidney transplantation outcomes from expanded criteria donors, standard criteria donors or living donors older than 60 years. Ren Fail. 2014; 36:526–533.
Article8. Kidney Disease Improving Global Outcomes. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012; 2:Suppl 1.9. Stratta RJ, Rohr MS, Sundberg AK, Armstrong G, Hairston G, Hartmann E, et al. Increased kidney transplantation utilizing expanded criteria deceased organ donors with results comparable to standard criteria donor transplant. Ann Surg. 2004; 239:688–695.
Article10. Sung RS, Guidinger MK, Lake CD, McBride MA, Greenstein SM, Delmonico FL, et al. Impact of the expanded criteria donor allocation system on the use of expanded criteria donor kidneys. Transplantation. 2005; 79:1257–1261.
Article11. Wynn JJ, Alexander CE. Increasing organ donation and transplantation: the U.S. experience over the past decade. Transpl Int. 2011; 24:324–332.
Article12. Kayler LK, Garzon P, Magliocca J, Fujita S, Kim RD, Hemming AW, et al. Outcomes and utilization of kidneys from deceased donors with acute kidney injury. Am J Transplant. 2009; 9:367–373.
Article13. Fraser SM, Rajasundaram R, Aldouri A, Farid S, Morris-Stiff G, Baker R, et al. Acceptable outcome after kidney transplantation using “expanded criteria donor” grafts. Transplantation. 2010; 89:88–96.
Article14. Serur D, Saal S, Wang J, Sullivan J, Bologa R, Hartono C, et al. Deceased-donor kidney transplantation: improvement in long-term survival. Nephrol Dial Transplant. 2011; 26:317–324.
Article15. La Manna G, Comai G, Cappuccilli ML, Liviano D'Arcangelo G, Fabbrizio B, Valentini C, et al. Prediction of three-year outcome of renal transplantation from optimal donors versus expanded criteria donors. Am J Nephrol. 2013; 37:158–166.
Article16. Ugarte R, Kraus E, Montgomery RA, Burdick JF, Ratner L, Haas M, et al. Excellent outcomes after transplantation of deceased donor kidneys with high terminal creatinine and mild pathologic lesions. Transplantation. 2005; 80:794–800.
Article17. Farney AC, Rogers J, Orlando G, al-Geizawi S, Buckley M, Farooq U, et al. Evolving experience using kidneys from deceased donors with terminal acute kidney injury. J Am Coll Surg. 2013; 216:645–655.
Article18. Jacobi J, Rebhan D, Heller K, Velden J, Hilgers KF, Wullich B, et al. Donor acute kidney injury and short-term graft outcome in renal transplantation. Clin Transplant. 2014; 28:1131–1141.
Article19. Heilman RL, Smith ML, Kurian SM, Huskey J, Batra RK, Chakkera HA, et al. Transplanting kidneys from deceased donors with severe acute kidney injury. Am J Transplant. 2015; 15:2143–2151.
Article20. Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transpl Int. 2014; 27:19–27.
Article21. Solini S, Aiello S, Cassis P, Scudeletti P, Azzollini N, Mister M, et al. Prolonged cold ischemia accelerates cellular and humoral chronic rejection in a rat model of kidney allotransplantation. Transpl Int. 2012; 25:347–356.
Article22. Ponticelli C. Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant. 2014; 29:1134–1140.
Article23. van der Vliet JA, Warlé MC, Cheung CL, Teerenstra S, Hoitsma AJ. Influence of prolonged cold ischemia in renal transplantation. Clin Transplant. 2011; 25:E612–E616.
Article24. Pérez Valdivia MA, Gentil MA, Toro M, Cabello M, Rodríguez-Benot A, Mazuecos A, et al. Impact of cold ischemia time on initial graft function and survival rates in renal transplants from deceased donors performed in Andalusia. Transplant Proc. 2011; 43:2174–2176.
Article25. Debout A, Foucher Y, Trébern-Launay K, Legendre C, Kreis H, Mourad G, et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015; 87:343–349.
Article26. Opelz G, Döhler B. Multicenter analysis of kidney preservation. Transplantation. 2007; 83:247–253.
Article27. Asderakis A, Dyer P, Augustine T, Worthington J, Campbell B, Johnson RW. Effect of cold ischemic time and HLA matching in kidneys coming from “young” and “old” donors: do not leave for tomorrow what you can do tonight. Transplantation. 2001; 72:674–678.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thrombotic microangiopathy, rare cause of deceased donor acute kidney injury: is a donor biopsy necessary before donation?
- Evaluation and Utilization of Expanded Criteria Dornor
- A Preliminary Study to Revise the Marginal Donor Criteria of KONOS in Deceased Donor Kidney Transplantation
- Impact of preoperative ultrasonography for predicting the prognosis of deceased donor kidney transplantation
- Outcome of living donor kidney transplant and deceased donor kidney transplant: a retrospective cohort study at national kidney and transplant institute