Yonsei Med J.
2017 Jul;58(4):770-777. 10.3349/ymj.2017.58.4.770.
Change in Renal Function among HIV-Infected Koreans Receiving Tenofovir Disoproxil Fumarate-Backbone Antiretroviral Therapy: A 3-Year Follow-Up Study
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. shhan74@yuhs.ac
- 2Division of Infectious Diseases, Department of Internal Medicine, Hongik Hospital, Seoul, Korea.
- KMID: 2419083
- DOI: http://doi.org/10.3349/ymj.2017.58.4.770
Abstract
- PURPOSE
Tenofovir disoproxil fumarate (TDF) is commonly prescribed as a fixed-dose, co-formulated antiretroviral drug for HIV-1 infection. The major concern of long-term TDF use is renal dysfunction. However, little is known about the long-term patterns of changes in renal function in HIV-infected Koreans receiving TDF.
MATERIALS AND METHODS
We prospectively followed 50 HIV-infected Koreans, performing laboratory tests every 3 months during the first year and every 6 months for the next 2 years. Urine N-acetyl-β-D-glucosaminidase (NAG) and plasma cystatin-C were measured using samples collected in the first year. Data on renal function were retrospectively collected on HIV-infected patients receiving first-line TDF (n=40) and in antiretroviral therapy (ART)-naïve patients (n=24) for 3 years. Renal function was evaluated as estimated glomerular filtration rate (eGFR) from serum creatinine [Modification of Diet in Renal Disease (MDRD)] and cystatin-C.
RESULTS
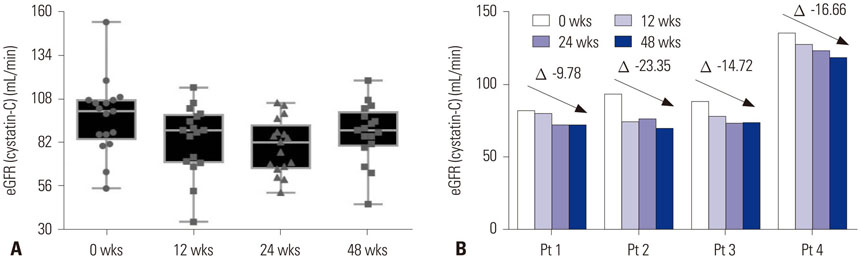
The eGFR (cystatin-C) showed significant changes from 0 to 48 wks (p=0.002), with the lowest levels at 24 wks (84.3±18.8 mL/min vs. 90.3±22.5 mL/min, p=0.021 by post hoc test). Urine NAG levels did not differ at 0, 12, 24, and 48 wks, although eGFR (MDRD) significantly decreased from 0 (98.7±18.9 mL/min/1.73 m²) to 144 wks (89.0±14.7 mL/min/1.73 m²) (p=0.010). The first-line TDF group had significantly lower eGFR (MDRD) than the ART-naïve group at 144 wks (89.7 mL/min/1.73 m² vs. 98.4 mL/min/1.73 m², p=0.036). Thirteen (26%) participants experienced a decrease in renal impairment of 10 mL/min/1.73 m² in eGFR (MDRD) at 144 wks.
CONCLUSION
These data suggest that clinically meaningful renal injury can develop in HIV-infected Koreans receiving long-term TDF.
Keyword
MeSH Terms
-
Adult
Anti-HIV Agents/pharmacology/*therapeutic use
Diet
Female
Follow-Up Studies
HIV Infections/*drug therapy/*physiopathology
HIV-1
Humans
Kidney/drug effects/*physiopathology
*Kidney Function Tests
Kidney Tubules/drug effects/physiopathology
Male
Tenofovir/pharmacology/*therapeutic use
Anti-HIV Agents
Tenofovir
Figure
Reference
-
1. Hemkens LG, Ewald H, Santini-Oliveira M, Bühler JE, Vuichard D, Schandelmaier S, et al. Comparative effectiveness of tenofovir in treatment-naïve HIV-infected patients: systematic review and meta-analysis. HIV Clin Trials. 2015; 16:178–189.
Article2. Prasitsuebsai W, Teeraananchai S, Singtoroj T, Truong KH, Ananworanich J, Do VC, et al. Treatment outcomes and resistance patterns of children and adolescents on second-line antiretroviral therapy in Asia. J Acquir Immune Defic Syndr. 2016; 72:380–386.
Article3. Brooks K, Diero L, DeLong A, Balamane M, Reitsma M, Kemboi E, et al. Treatment failure and drug resistance in HIV-positive patients on tenofovir-based first-line antiretroviral therapy in western Kenya. J Int AIDS Soc. 2016; 19:20798.
Article4. Labhardt ND, Bader J, Lejone TI, Ringera I, Puga D, Glass TR, et al. Is zidovudine first-line therapy virologically comparable to tenofovir in resource-limited settings? Trop Med Int Health. 2015; 20:914–918.
Article5. Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004; 292:191–201.
Article6. Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol. 2013; 24:1519–1527.
Article7. Casado JL. Renal and bone toxicity with the use of tenofovir: understanding at the end. AIDS Rev. 2016; 18:59–68.8. Del Palacio M, Romero S, Casado JL. Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev. 2012; 14:179–187.9. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011; 57:773–780.
Article10. Kohler JJ, Hosseini SH, Hoying-Brandt A, Green E, Johnson DM, Russ R, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest. 2009; 89:513–519.
Article11. Lebrecht D, Venhoff AC, Kirschner J, Wiech T, Venhoff N, Walker UA. Mitochondrial tubulopathy in tenofovir disoproxil fumaratetreated rats. J Acquir Immune Defic Syndr. 2009; 51:258–263.
Article12. Ramamoorthy H, Abraham P, Isaac B. Mitochondrial dysfunction and electron transport chain complex defect in a rat model of tenofovir disoproxil fumarate nephrotoxicity. J Biochem Mol Toxicol. 2014; 28:246–255.
Article13. Nishijima T, Komatsu H, Higasa K, Takano M, Tsuchiya K, Hayashida T, et al. Single nucleotide polymorphisms in ABCC2 associate with tenofovir-induced kidney tubular dysfunction in Japanese patients with HIV-1 infection: a pharmacogenetic study. Clin Infect Dis. 2012; 55:1558–1567.
Article14. Rodríguez-Nóvoa S, Labarga P, Soriano V, Egan D, Albalater M, Morello J, et al. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis. 2009; 48:e108–e116.
Article15. Dauchy FA, Lawson-Ayayi S, de La Faille R, Bonnet F, Rigothier C, Mehsen N, et al. Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy. Kidney Int. 2011; 80:302–309.
Article16. Labarga P, Barreiro P, Martin-Carbonero L, Rodriguez-Novoa S, Solera C, Medrano J, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS. 2009; 23:689–696.
Article17. Andrade-Fuentes K, Mata-Marín JA, López-De León JI, Manjarrez-Téllez B, Ramírez JL, Gaytan-Martínez J. Proximal renal tubular dysfunction related to antiretroviral therapy among HIV-infected patients in an HIV clinic in Mexico. AIDS Patient Care STDS. 2015; 29:181–185.
Article18. Nishijima T, Komatsu H, Gatanaga H, Aoki T, Watanabe K, Kinai E, et al. Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients. PLoS One. 2011; 6:e22661.
Article19. Nishijima T, Gatanaga H, Komatsu H, Tsukada K, Shimbo T, Aoki T, et al. Renal function declines more in tenofovir- than abacavirbased antiretroviral therapy in low-body weight treatment-naïve patients with HIV infection. PLoS One. 2012; 7:e29977.
Article20. Nishijima T, Kawasaki Y, Tanaka N, Mizushima D, Aoki T, Watanabe K, et al. Long-term exposure to tenofovir continuously decrease renal function in HIV-1-infected patients with low body weight: results from 10 years of follow-up. AIDS. 2014; 28:1903–1910.
Article21. Quesada PR, Esteban LL, García JR, Sánchez RV, García TM, Alonso-Vega GG, et al. Incidence and risk factors for tenofovir-associated renal toxicity in HIV-infected patients. Int J Clin Pharm. 2015; 37:865–872.
Article22. Rokx C, Van der Ende ME, Rijnders BJ. How does weight influence tenofovir disoproxil-fumarate induced renal function decline? AIDS. 2015; 29:643–645.
Article23. Onopiuk A, Tokarzewicz A, Gorodkiewicz E. Cystatin C: a kidney function biomarker. Adv Clin Chem. 2015; 68:57–69.24. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–163.25. Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011; 35:561–566.
Article26. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145:247–254.
Article27. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004; 64:25–30.
Article28. Nishijima T, Shimbo T, Komatsu H, Takano M, Tanuma J, Tsukada K, et al. Urinary beta-2 microglobulin and alpha-1 microglobulin are useful screening markers for tenofovir-induced kidney tubulopathy in patients with HIV-1 infection: a diagnostic accuracy study. J Infect Chemother. 2013; 19:850–857.
Article29. Roldán V, Marín F, Fernández H, Manzano-Fernández S, Gallego P, Valdés M, et al. Renal impairment in a “real-life” cohort of anticoagulated patients with atrial fibrillation (implications for thromboembolism and bleeding). Am J Cardiol. 2013; 111:1159–1164.
Article30. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005; 67:2089–2100.
Article31. Kyaw NT, Harries AD, Chinnakali P, Antierens A, Soe KP, Woodman M, et al. Low incidence of renal dysfunction among HIV-infected patients on a tenofovir-based first line antiretroviral treatment regimen in myanmar. PLoS One. 2015; 10:e0135188.
Article32. Pujari SN, Smith C, Makane A, Youle M, Johnson M, Bele V, et al. Higher risk of renal impairment associated with tenofovir use amongst people living with HIV in India: a comparative cohort analysis between Western India and United Kingdom. BMC Infect Dis. 2014; 14:173.
Article33. Chua AC, Llorin RM, Lai K, Cavailler P, Law HL. Renal safety of tenofovir containing antiretroviral regimen in a Singapore cohort. AIDS Res Ther. 2012; 9:19.
Article34. Mizushima D, Tanuma J, Dung NT, Dung NH, Trung NV, Lam NT, et al. Low body weight and tenofovir use are risk factors for renal dysfunction in Vietnamese HIV-infected patients. A prospective 18-month observation study. J Infect Chemother. 2014; 20:784–788.
Article35. Chaisiri K, Bowonwatanuwong C, Kasettratat N, Kiertiburanakul S. Incidence and risk factors for tenofovir-associated renal function decline among Thai HIV-infected patients with low-body weight. Curr HIV Res. 2010; 8:504–509.
Article36. Stevens LA, Padala S, Levey AS. Advances in glomerular filtration rate-estimating equations. Curr Opin Nephrol Hypertens. 2010; 19:298–307.
Article37. Chew-Harris JS, Florkowski CM, George PM, Elmslie JL, Endre ZH. The relative effects of fat versus muscle mass on cystatin C and estimates of renal function in healthy young men. Ann Clin Biochem. 2013; 50(Pt 1):39–46.
Article38. Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009; 75:652–660.
Article39. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012; 367:20–29.
Article40. Longenecker CT, Kitch D, Sax PE, Daar ES, Tierney C, Gupta SK, et al. Reductions in plasma cystatin C after initiation of antiretroviral therapy are associated with reductions in inflammation: ACTG A5224s. J Acquir Immune Defic Syndr. 2015; 69:168–177.
Article41. Schley G, Köberle C, Manuilova E, Rutz S, Forster C, Weyand M, et al. Comparison of plasma and urine biomarker performance in acute kidney injury. PLoS One. 2015; 10:e0145042.
Article42. Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. 2014; 29:1301–1311.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recovery of Tenofovir-induced Nephrotoxicity following Switch from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in Human Immunodeficiency Virus-Positive Patients
- Comparative pharmacokinetics between tenofovir disoproxil phosphate and tenofovir disoproxil fumarate in healthy subjects
- Letter: Cardiovascular risk of tenofovir disoproxil fumarate or tenofovir alafenamide fumarate in patients with chronic hepatitis B: More questions than an answer – author’s reply
- Incidence and Risk Factors of Tenofovir Disoproxil Fumarate Induced Nephrotoxicity and Renal Function Recovery, a Hospital Case-Control Study
- Incidence and risk factors for tenofovir-associated nephrotoxicity among human immunodeficiency virus-infected patients in Korea