Yonsei Med J.
2017 Sep;58(5):1012-1017. 10.3349/ymj.2017.58.5.1012.
Endothelial Progenitor Cells Correlated with Oxidative Stress after Mild Traumatic Brain Injury
- Affiliations
-
- 1Department of Neurosurgery, Shanxi Medical University First Affiliated Hospital, Taiyuan, China. xintaoh@hotmail.com
- 2Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, China.
- KMID: 2418939
- DOI: http://doi.org/10.3349/ymj.2017.58.5.1012
Abstract
- PURPOSE
Endothelial progenitor cells (EPCs) play a key role in tissue repair and regeneration. Previous studies have shown that infusion of human umbilical cord blood-derived endothelial colony-forming cells improves outcomes in mice subjected to experimental traumatic brain injury (TBI). However, the efficiency of cell transplantation is not satisfactory. Oxidative stress plays a significant role in the survival of transplanted cells following ischemic reperfusion injury. This observational clinical study investigated the correlation between the number of circulating EPCs and plasma levels of superoxide dismutase (SOD) and malonyldialdehyde (MDA).
MATERIALS AND METHODS
Peripheral blood samples were collected from 20 patients with mild TBI at day-1, day-2, day-3, day-4, and day-7 post TBI. The number of circulating EPCs and the plasma levels of SOD and MDA were measured.
RESULTS
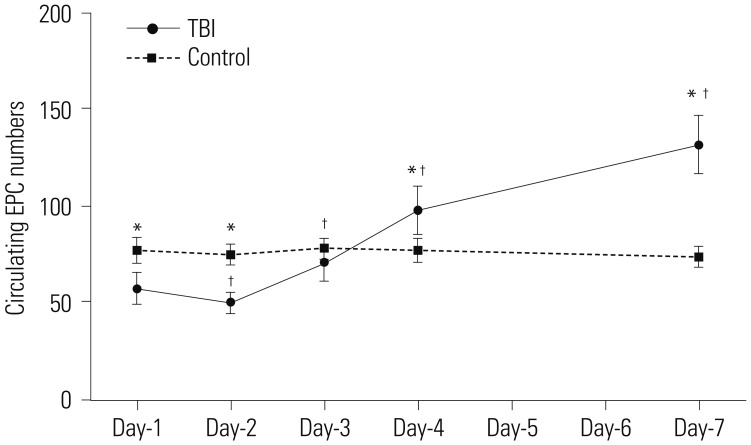
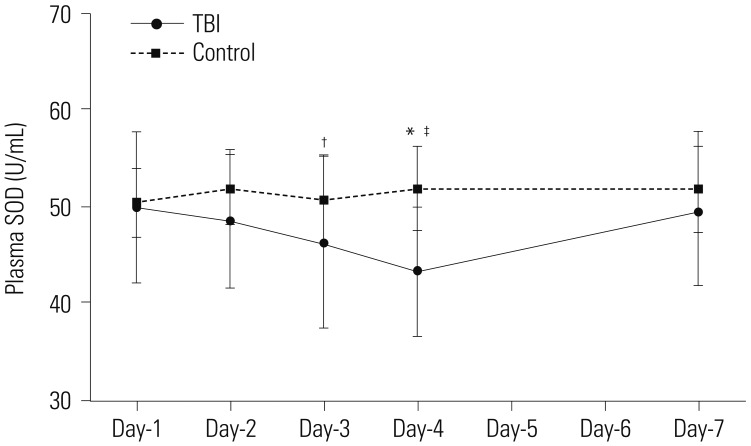
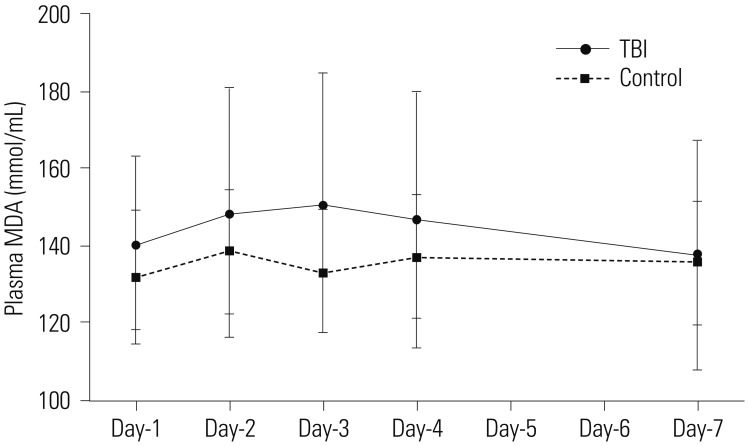
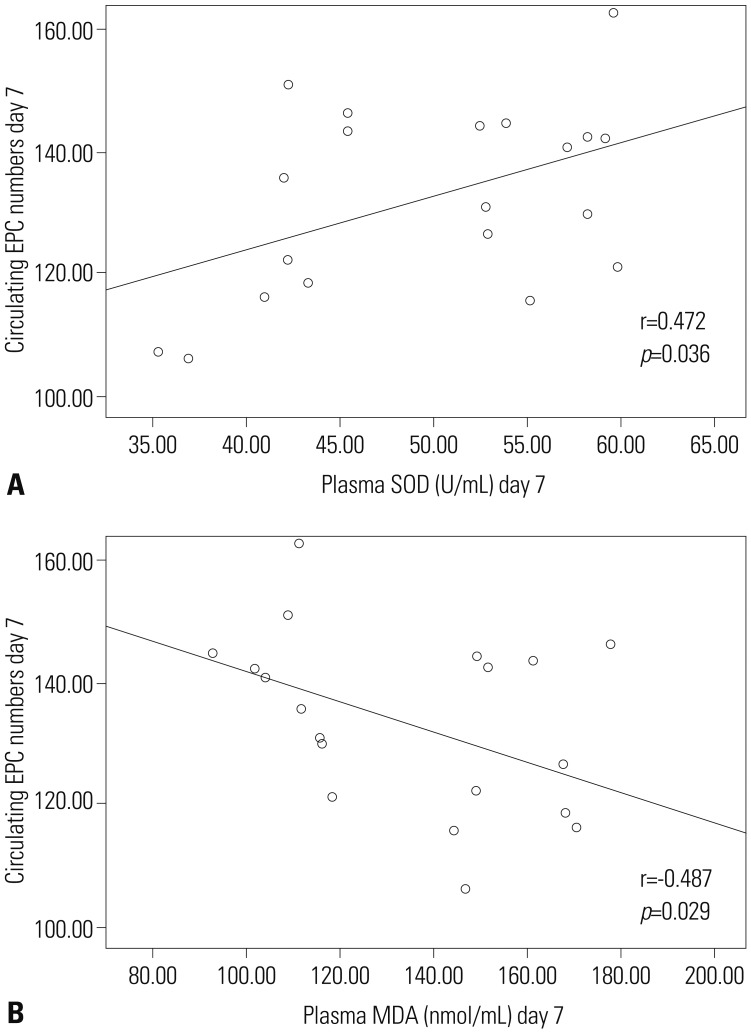
The average of circulating EPCs in TBI patients decreased initially, but increased thereafter, compared with healthy controls. Plasma levels of SOD in TBI patients were significantly lower than those in healthy controls at day-4 post-TBI. MDA levels showed no difference between the two groups. Furthermore, when assessed on day-7 post-TBI, the circulating EPC number were correlated with the plasma levels of SOD and MDA.
CONCLUSION
These results suggest that the number of circulating EPCs is weakly to moderately correlated with plasma levels of SOD and MDA at day-7 post-TBI, which may offer a novel antioxidant strategy for EPCs transplantation after TBI.
Keyword
MeSH Terms
Figure
Reference
-
1. Mendes Arent A, de Souza LF, Walz R, Dafre AL. Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed Res Int. 2014; 2014:723060. PMID: 24689052.
Article2. Rodríguez-Rodríguez A, Egea-Guerrero JJ, Murillo-Cabezas F, Carrillo-Vico A. Oxidative stress in traumatic brain injury. Curr Med Chem. 2014; 21:1201–1211. PMID: 24350853.3. Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010; 11:298–308.4. Hayward NM, Immonen R, Tuunanen PI, Ndode-Ekane XE, GrÖhn O, Pitkänen A. Association of chronic vascular changes with functional outcome after traumatic brain injury in rats. J Neurotrauma. 2010; 27:2203–2219. PMID: 20839948.
Article5. Briasoulis A, Tousoulis D, Antoniades C, Papageorgiou N, Stefanadis C. The role of endothelial progenitor cells in vascular repair after arterial injury and atherosclerotic plaque development. Cardiovasc Ther. 2011; 29:125–139. PMID: 20406237.
Article6. Liu L, Wei H, Chen F, Wang J, Dong JF, Zhang J. Endothelial progenitor cells correlate with clinical outcome of traumatic brain injury. Crit Care Med. 2011; 39:1760–1765. PMID: 21460712.
Article7. Liu L, Liu H, Jiao J, Liu H, Bergeron A, Dong JF, et al. Changes in circulating human endothelial progenitor cells after brain injury. J Neurotrauma. 2007; 24:936–943. PMID: 17600511.
Article8. Huang XT, Zhang YQ, Li SJ, Li SH, Tang Q, Wang ZT, et al. Intracerebroventricular transplantation of ex vivo expanded endothelial colony-forming cells restores blood-brain barrier integrity and promotes angiogenesis of mice with traumatic brain injury. J Neurotrauma. 2013; 30:2080–2088. PMID: 23957220.9. Zhang Y, Li Y, Wang S, Han Z, Huang X, Li S, et al. Transplantation of expanded endothelial colony-forming cells improved outcomes of traumatic brain injury in a mouse model. J Surg Res. 2013; 185:441–449. PMID: 23953790.
Article10. Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, et al. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009; 27:2185–2195. PMID: 19557831.
Article11. Monsel A, Zhu YG, Gennai S, Hao Q, Liu J, Lee JW. Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology. 2014; 121:1099–1121. PMID: 25211170.12. Gennai S, Monsel A, Hao Q, Liu J, Gudapati V, Barbier EL, et al. Cell-based therapy for traumatic brain injury. Br J Anaesth. 2015; 115:203–212. PMID: 26170348.
Article13. Abdul-Muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2015; 51:966–979. PMID: 24865512.
Article14. Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, et al. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 2013; 60:282–291. PMID: 23466554.
Article15. Mojtahedzadeh M, Ahmadi A, Mahmoodpoor A, Beigmohammadi MT, Abdollahi M, Khazaeipour Z, et al. Hypertonic saline solution reduces the oxidative stress responses in traumatic brain injury patients. J Res Med Sci. 2014; 19:867–874. PMID: 25535502.16. Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010; 7:51–61. PMID: 20129497.
Article17. Kochanek PM, Jackson TC, Ferguson NM, Carlson SW, Simon DW, Brockman EC, et al. Emerging therapies in traumatic brain injury. Semin Neurol. 2015; 35:83–100. PMID: 25714870.
Article18. Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, Pennisi G, et al. Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal. 2013; 19:836–853. PMID: 23547621.
Article19. Cernak I, Savic VJ, Kotur J, Prokic V, Veljovic M, Grbovic D. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000; 17:53–68. PMID: 10674758.
Article20. Lu XY, Wang HD, Xu JG, Ding K, Li T. NADPH oxidase inhibition improves neurological outcome in experimental traumatic brain injury. Neurochem Int. 2014; 69:14–19. PMID: 24589771.
Article21. Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res. 2002; 51:571–578. PMID: 11978879.
Article22. Liao Y, Liu P, Guo F, Zhang ZY, Zhang Z. Oxidative burst of circulating neutrophils following traumatic brain injury in human. PLoS One. 2013; 8:e68963. PMID: 23894384.
Article23. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275:964–967. PMID: 9020076.
Article24. Yu P, Zhang Z, Li S, Wen X, Quan W, Tian Q, et al. Progesterone modulates endothelial progenitor cell (EPC) viability through the CXCL12/CXCR4/PI3K/Akt signalling pathway. Cell Prolif. 2016; 49:48–57. PMID: 26818151.
Article25. Shen LH, Ye M, Ding XS, Han Q, Zhang C, Liu XF, et al. Protective effects of MCI-186 on transplantation of bone marrow stromal cells in rat ischemic stroke model. Neuroscience. 2012; 223:315–324. PMID: 22885235.
Article26. Fukui M, Zhu BT. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic Biol Med. 2010; 48:821–830. PMID: 20060889.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Store-Operated Calcium Entry Protects Endothelial Progenitor Cells from Hâ‚‚Oâ‚‚-Induced Apoptosis
- Glutathione Protects Brain Endothelial Cells from Hydrogen Peroxide-Induced Oxidative Stress by Increasing Nrf2 Expression
- Diagnostic History of Traumatic Axonal Injury in Patients with Cerebral Concussion and Mild Traumatic Brain Injury
- Tea Flavonoids Induced Differentiation of Peripheral Blood-derived Mononuclear Cells into Peripheral Blood-derived Endothelial Progenitor Cells and Suppressed Intracellular Reactive Oxygen Species Level of Peripheral Blood-derived Endothelial Progenitor Cells
- Correlation between NADPH oxidase-mediated oxidative stress and dysfunction of endothelial progenitor cell in hyperlipidemic patients