Yonsei Med J.
2018 Mar;59(2):341-344. 10.3349/ymj.2018.59.2.341.
Olmsted Syndrome Caused by a Heterozygous p.Gly568Val Missense Mutation in TRPV3 Gene
- Affiliations
-
- 1Department of Dermatology, Cutaneous Biology Research Institute, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. kimsc@yuhs.ac
- KMID: 2418801
- DOI: http://doi.org/10.3349/ymj.2018.59.2.341
Abstract
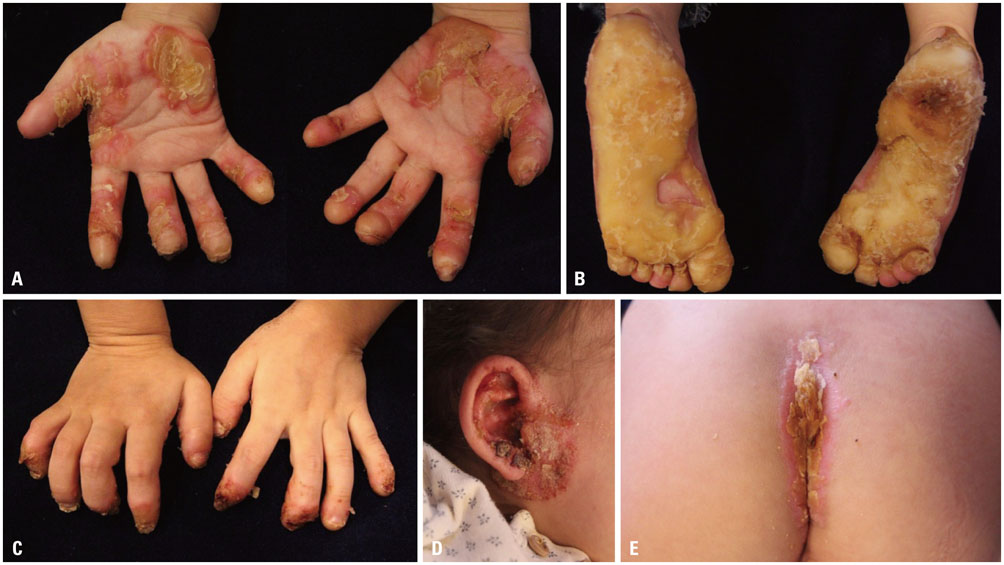
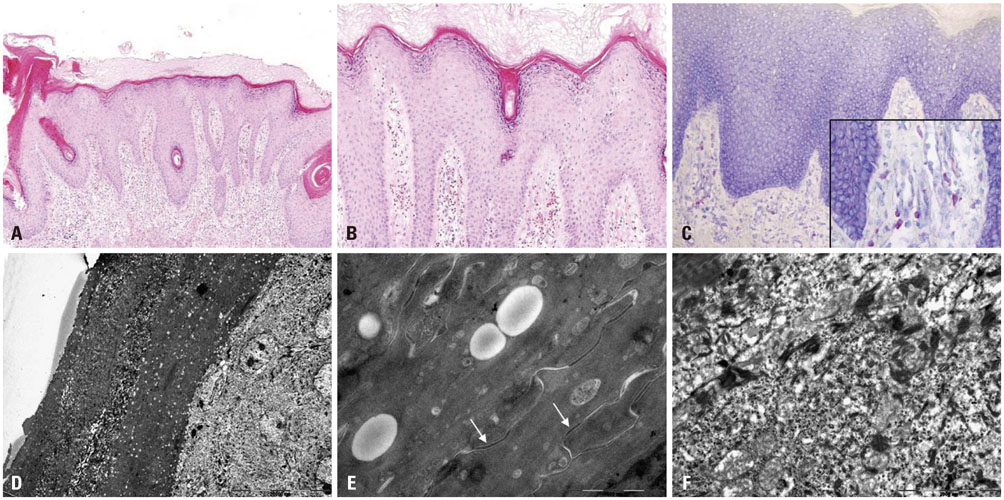
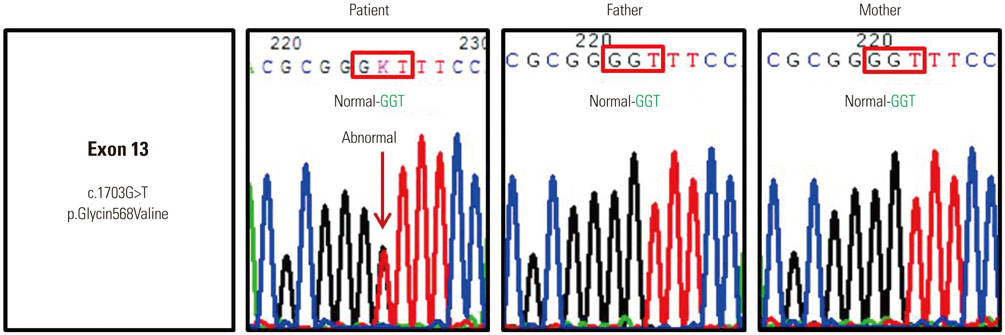
- Olmsted syndrome (OS) is a rare congenital skin disorder characterized by severe palmoplantar and periorificial keratoderma, alopecia, onychodystrophy, and severe pruritus. Recently, pathogenic "˜gain-of-function"˜ mutations of the transient receptor potential vanilloid 3 gene (TRPV3), which encodes a cation channel involved in keratinocyte differentiation and proliferation, hair growth, inflammation, pain and pruritus, have been identified to cause OS. Due to the rarity, the pattern of inheritance of OS is still unclear. We report a case of OS in a 3-year-old Korean girl and its underlying gene mutation. The patient presented with a disabling, bilateral palmoplantar keratoderma with onychodystrophy. She also exhibited pruritic eczematous skin lesions around her eyes, ears and gluteal fold. Genetic analysis identified a heterozygous p.Gly568Val missense mutation in the exon 13 of TRPV3. To our knowledge, this is the first case of OS in the Korean population showing a missense mutation p.Gly573Ser.
Keyword
MeSH Terms
Figure
Reference
-
1. Olmsted HC. Keratodermia palmaris et plantaris congenitalis: report of a case showing associated lesions of unusual location. Am J Dis Child. 1927; 33:757–764.2. Atherton DJ, Sutton C, Jones BM. Mutilating palmoplantar keratoderma with periorificial keratotic plaques (Olmsted’s syndrome). Br J Dermatol. 1990; 122:245–252.
Article3. Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015; 10:33.
Article4. Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012; 90:558–564.
Article5. He Y, Zeng K, Zhang X, Chen Q, Wu J, Li H, et al. A gain-of-function mutation in TRPV3 causes focal palmoplantar keratoderma in a Chinese family. J Invest Dermatol. 2015; 135:907–909.
Article6. Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002; 418:181–186.
Article7. Eytan O, Fuchs-Telem D, Mevorach B, Indelman M, Bergman R, Sarig O, et al. Olmsted syndrome caused by a homozygous recessive mutation in TRPV3. J Invest Dermatol. 2014; 134:1752–1754.
Article8. Duchatelet S, Guibbal L, de Veer S, Fraitag S, Nitschké P, Zarhrate M, et al. Olmsted syndrome with erythromelalgia caused by recessive transient receptor potential vanilloid 3 mutations. Br J Dermatol. 2014; 171:675–678.
Article9. Agarwala MK, George R, Pramanik R, McGrath JA. Olmsted syndrome in an Indian male with a new de novo mutation in TRPV3. Br J Dermatol. 2016; 174:209–211.
Article10. Wilson NJ, Cole C, Milstone LM, Kiszewski AE, Hansen CD, O'Toole EA, et al. Expanding the phenotypic spectrum of Olmsted syndrome. J Invest Dermatol. 2015; 135:2879–2883.
Article11. Nagai H, Takaoka Y, Sugano A, Nakamachi Y, Kawano S, Nishigori C. Identification of a heterozygous p.Gly568Val missense mutation in the TRPV3 gene in a Japanese patient with Olmsted syndrome: in silico analysis of TRPV3. J Dermatol. 2017; 44:1059–1062.
Article12. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424.
Article13. Borbíró I, Lisztes E, Tóth BI, Czifra G, Oláh A, Szöllosi AG, et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J Invest Dermatol. 2011; 131:1605–1614.
Article14. Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Sorèze 2009. J Am Acad Dermatol. 2010; 63:607–641.
Article15. Fartasch M, Williams ML, Elias PM. Altered lamellar body secretion and stratum corneum membrane structure in Netherton syndrome: differentiation from other infantile erythrodermas and pathogenic implications. Arch Dermatol. 1999; 135:823–832.
Article16. Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br J Pharmacol. 2012; 165:683–692.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A compound heterozygous mutation in the FMO3 gene: the first pediatric case causes fish odor syndrome in Korea
- A family with X-linked Cornelia de Lange syndrome due to a novel SMC1A missense mutation identified by multi-gene panel sequencing
- A Family with A Missense Mutation in the SCN5A Gene
- Early onset of colorectal cancer in a 13-year-old girl with Lynch syndrome
- Polymorphism of UDP-glucuronosyltransferase Gene(UGT1A1) of Neonatal Hyperbilirubinemia in Korea