Yonsei Med J.
2018 Mar;59(2):219-225. 10.3349/ymj.2018.59.2.219.
Prostate-Specific Antigen Kinetics Following 5α-Reductase Inhibitor Treatment May Be a Useful Indicator for Repeat Prostate Biopsy
- Affiliations
-
- 1Department of Urology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. winner0428@gmail.com
- 2Department of Urology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Urology, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- KMID: 2418784
- DOI: http://doi.org/10.3349/ymj.2018.59.2.219
Abstract
- PURPOSE
To evaluate parameters for determining repeat prostate biopsy in patients with 5α-reductase inhibitor (5ARI) treatment after initial negative biopsy.
MATERIALS AND METHODS
From January 2007 to December 2015, patients who underwent a repeat prostate biopsy after an initial negative biopsy were enrolled from multiple institutions. Serial prostate-specific antigen (PSA) levels after the initial biopsy were analyzed for PSA kinetics. Clinicopathologic variables were evaluated according to the use of 5ARIs after the initial negative biopsy.
RESULTS
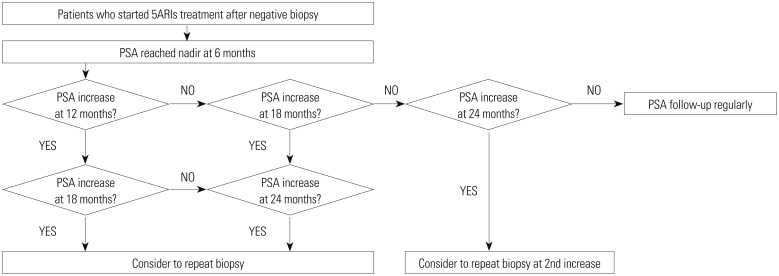
Of 419 patients with initial negative biopsies (median age=67.0 years, median PSA=6.31 ng/mL), 101 patients (24.1%) were diagnosed with prostate cancer at the repeat biopsy. An increase in PSA level at 18 months, compared to that at 6 months, was a predictor of a positive repeat biopsy. However, the use of 5ARIs was not identified as a predictor. Of 126 patients receiving 5ARI treatment after the initial biopsy, 30 (23.8%) were diagnosed with prostate cancer at the repeat biopsy. Increase in PSA level at more than two time points after 6 months of 5ARI treatment (odds ratio=4.84, p=0.005) was associated with cancer detection at the repeat biopsy. There were no significant 5ARI group-related differences in the detection rates of prostate and high-grade cancers (Gleason score ≥7).
CONCLUSION
The effects of 5ARIs on prostate cancer detection and chemoprevention remain uncertain. However, more than two increases in PSA level after 6 months of 5ARI treatment may indicate the presence of prostate cancer.
MeSH Terms
Figure
Reference
-
1. Schröder FH. Landmarks in prostate cancer screening. BJU Int. 2012; 110(Suppl 1):3–7. PMID: 23046034.
Article2. Basch E, Oliver TK, Vickers A, Thompson I, Kantoff P, Parnes H, et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology Provisional Clinical Opinion. J Clin Oncol. 2012; 30:3020–3025. PMID: 22802323.
Article3. Presti JC Jr, O’Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003; 169:125–129. PMID: 12478119.
Article4. Koo KC, Lee DH, Lee SH, Chung BH. Peripheral zone prostate-specific antigen density: an effective parameter for prostate cancer prediction in men receiving 5α-reductase inhibitors. Prostate Int. 2013; 1:102–108. PMID: 24223410.
Article5. Choi YH, Cho SY, Cho IR. The different reduction rate of prostate-specific antigen in dutasteride and finasteride. Korean J Urol. 2010; 51:704–708. PMID: 21031091.
Article6. Aganovic D, Prcic A, Kulovac B, Hadziosmanovic O. Influence of the prostate volume, prostate specific antigen density and number of biopsy samples on prostate cancer detection. Med Arh. 2012; 66:41–44. PMID: 22482342.
Article7. Qi TY, Chen YQ, Jiang J, Zhu YK, Yao XH, Wang XJ. Utility of the transition zone index for identification of prostate cancer in Chinese men with intermediate PSA levels. Int Urol Nephrol. 2012; 44:807–815. PMID: 22311386.
Article8. Vickers AJ, Sjoberg DD. Decision analysis of dutasteride use for patients with negative prostate biopsy. Urology. 2015; 85:337–341. PMID: 25623680.
Article9. Oderda M, Zitella A, Richiardi L, Tizzani A, Gontero P. Effect of finasteride on the sensitivity of PSA to detect prostate cancer in rebiopsy series. Arch Ital Urol Androl. 2010; 82:135–138.10. Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003; 349:215–224. PMID: 12824459.
Article11. Thompson IM Jr, Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013; 369:603–610. PMID: 23944298.
Article12. Pinsky PF, Black A, Grubb R, Crawford ED, Andriole G, Thompson I, et al. Projecting prostate cancer mortality in the PCPT and REDUCE chemoprevention trials. Cancer. 2013; 119:593–601. PMID: 22893105.
Article13. Roehrborn CG, Andriole GL, Wilson TH, Castro R, Rittmaster RS. Effect of dutasteride on prostate biopsy rates and the diagnosis of prostate cancer in men with lower urinary tract symptoms and enlarged prostates in the Combination of Avodart and Tamsulosin trial. Eur Urol. 2011; 59:244–249. PMID: 21093145.
Article14. Andriole GL, Guess HA, Epstein JI, Wise H, Kadmon D, Crawford ED, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1998; 52:195–201. PMID: 9697781.15. Redman MW, Tangen CM, Goodman PJ, Lucia MS, Coltman CA Jr, Thompson IM. Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach. Cancer Prev Res (Phila). 2008; 1:174–181. PMID: 19138953.
Article16. Kim SJ, Jeong TY, Yoo DS, Park J, Cho S, Kang SH, et al. Can prostate-specific antigen kinetics before prostate biopsy predict the malignant potential of prostate cancer. Yonsei Med J. 2015; 56:1492–1496. PMID: 26446628.
Article17. Celhay O, de la Taille A, Salomon L, Doré B, Irani J. Fluctuating prostate-specific antigen levels in patients with initial negative biopsy: should we be reassured. BJU Int. 2007; 99:1028–1030. PMID: 17324221.
Article18. Carter HB, Kettermann A, Ferrucci L, Landis P, Metter EJ. Prostate-specific antigen velocity risk count assessment: a new concept for detection of life-threatening prostate cancer during window of curability. Urology. 2007; 70:685–690. PMID: 17991538.
Article19. Ulmert D, Serio AM, O'Brien MF, Becker C, Eastham JA, Scardino PT, et al. Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol. 2008; 26:835–841. PMID: 18281654.
Article20. Marberger M, Freedland SJ, Andriole GL, Emberton M, Pettaway C, Montorsi F, et al. Usefulness of prostate-specific antigen (PSA) rise as a marker of prostate cancer in men treated with dutasteride: lessons from the REDUCE study. BJU Int. 2012; 109:1162–1169. PMID: 21699645.
Article21. van Leeuwen PJ, Kölble K, Huland H, Hambrock T, Barentsz J, Schröder FH. Prostate cancer detection and dutasteride: utility and limitations of prostate-specific antigen in men with previous negative biopsies. Eur Urol. 2011; 59:183–190. PMID: 21130560.
Article22. Lee KS, Koo KC, Cho KS, Lee SH, Han WK, Choi YD, et al. Indications for a second prostate biopsy in patients suspected with prostate cancer after an initial negative prostate biopsy. Prostate Int. 2017; 5:24–28. PMID: 28352620.
Article23. Andriole GL, Humphrey P, Ray P, Gleave ME, Trachtenberg J, Thomas LN, et al. Effect of the dual 5alpha-reductase inhibitor dutasteride on markers of tumor regression in prostate cancer. J Urol. 2004; 172:915–919. PMID: 15310997.24. Andriole GL, Marberger M, Roehrborn CG. Clinical usefulness of serum prostate specific antigen for the detection of prostate cancer is preserved in men receiving the dual 5alpha-reductase inhibitor dutasteride. J Urol. 2006; 175:1657–1662. PMID: 16600723.25. Brawer MK, Lin DW, Williford WO, Jones K, Lepor H. Effect of finasteride and/or terazosin on serum PSA: results of VA Cooperative Study #359. Prostate. 1999; 39:234–239. PMID: 10344212.
Article26. Kravchick S, Lobik L, Cytron S, Kravchenko Y, Dor DB, Peled R. Patients with persistently elevated PSA and negative results of TRUS-Biopsy: does 6-month treatment with dutasteride can indicate candidates for re-biopsy. what is the best of saturation schemes: transrectal or transperineal approach. Pathol Oncol Res. 2015; 21:985–989. PMID: 25753982.
Article27. van Houten ME, Gooren LJ. Differences in reproductive endocrinology between Asian men and Caucasian men--a literature review. Asian J Androl. 2000; 2:13–20. PMID: 11228931.28. Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG. The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol. 2006; 176:868–874. PMID: 16890642.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Predictive Factors of Prostatic Cancer Detection on Repeat Prostate Biopsy
- High-Grade Prostatic Intraepithelial Neoplasia
- The Efficacy of Combination Therapy of 5 alpha -Reductase Inhibitor and of-Adrenergic Blocker in Benign Prostate Hyperplasia
- Role of Prostate-Specific Antigen Change Ratio at Initial Biopsy as a Novel Decision-Making Marker for Repeat Prostate Biopsy
- The Diagnostic Value of Prostate-specific Antigen and the of Routine Laboratory Examination for Early Detection