Yonsei Med J.
2018 Mar;59(2):176-186. 10.3349/ymj.2018.59.2.176.
Systems Biology-Based Platforms to Accelerate Research of Emerging Infectious Diseases
- Affiliations
-
- 1Department of Biomedical Sciences, College of Medicine, Korea University Guro Hospital, Seoul, Korea. oshin@korea.ac.kr
- 2College of Medicine and Medical Research Institute, Chungbuk National University, Cheongju, Korea.
- KMID: 2418779
- DOI: http://doi.org/10.3349/ymj.2018.59.2.176
Abstract
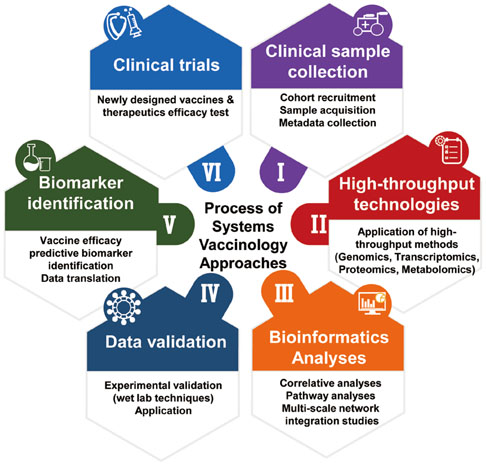
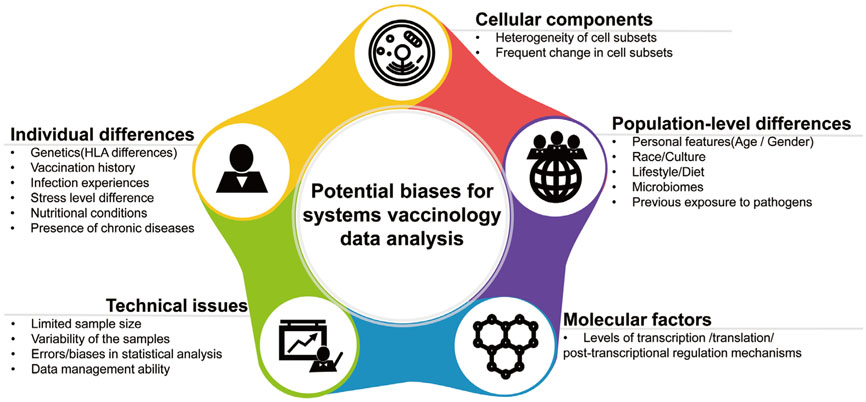
- Emerging infectious diseases (EIDs) pose a major threat to public health and security. Given the dynamic nature and significant impact of EIDs, the most effective way to prevent and protect against them is to develop vaccines in advance. Systems biology approaches provide an integrative way to understand the complex immune response to pathogens. They can lead to a greater understanding of EID pathogenesis and facilitate the evaluation of newly developed vaccine-induced immunity in a timely manner. In recent years, advances in high throughput technologies have enabled researchers to successfully apply systems biology methods to analyze immune responses to a variety of pathogens and vaccines. Despite recent advances, computational and biological challenges impede wider application of systems biology approaches. This review highlights recent advances in the fields of systems immunology and vaccinology, and presents ways that systems biology-based platforms can be applied to accelerate a deeper understanding of the molecular mechanisms of immunity against EIDs.
MeSH Terms
Figure
Reference
-
1. Morse SS, Schluederberg A. From the National Institute of Allergy and Infectious Diseases, the Fogarty International Center of the National Institutes of Health, and the Rockefeller University. Emerging viruses: the evolution of viruses and viral diseases. J Infect Dis. 1990; 162:1–7.2. Morse SS, Hughes JM. Developing an integrated epidemiologic approach to emerging infectious diseases. Epidemiol Rev. 1996; 18:1–3.
Article3. Woolhouse M, Scott F, Hudson Z, Howey R, Chase-Topping M. Human viruses: discovery and emergence. Philos Trans R Soc Lond B Biol Sci. 2012; 367:2864–2871.
Article4. Woolhouse ME. Population biology of emerging and re-emerging pathogens. Trends Microbiol. 2002; 10:10 Suppl. S3–S7.
Article5. Woolhouse ME, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005; 11:1842–1847.
Article6. Grimm SK, Ackerman ME. Vaccine design: emerging concepts and renewed optimism. Curr Opin Biotechnol. 2013; 24:1078–1088.
Article7. Nakaya HI, Pulendran B. Vaccinology in the era of high-throughput biology. Philos Trans R Soc Lond B Biol Sci. 2015; 370:20140146.
Article8. Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, et al. Accelerating next-generation vaccine development for global disease prevention. Science. 2013; 340:1232910.
Article9. Westerhoff HV, Winder C, Messiha H, Simeonidis E, Adamczyk M, Verma M, et al. Systems biology: the elements and principles of life. FEBS Lett. 2009; 583:3882–3890.
Article10. Nakaya HI, Li S, Pulendran B. Systems vaccinology: learning to compute the behavior of vaccine induced immunity. Wiley Interdiscip Rev Syst Biol Med. 2012; 4:193–205.
Article11. Li S, Nakaya HI, Kazmin DA, Oh JZ, Pulendran B. Systems biological approaches to measure and understand vaccine immunity in humans. Semin Immunol. 2013; 25:209–218.
Article12. Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010; 33:516–529.
Article13. Pulendran B, Oh JZ, Nakaya HI, Ravindran R, Kazmin DA. Immunity to viruses: learning from successful human vaccines. Immunol Rev. 2013; 255:243–255.
Article14. Almeida SL. Trending now: re-emerging infectious disease update. J Emerg Nurs. 2015; 41:104–108.
Article15. Howard CR, Fletcher NF. Emerging virus diseases: can we ever expect the unexpected? Emerg Microbes Infect. 2012; 1:e46.
Article16. Dash AP, Bhatia R, Sunyoto T, Mourya DT. Emerging and re-emerging arboviral diseases in Southeast Asia. J Vector Borne Dis. 2013; 50:77–84.17. Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995; 1:7–15.
Article18. Lauring AS, Frydman J, Andino R. The role of mutational robustness in RNA virus evolution. Nat Rev Microbiol. 2013; 11:327–336.
Article19. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56:152–179.
Article20. Yoon SW, Webby RJ, Webster RG. Evolution and ecology of influenza A viruses. Curr Top Microbiol Immunol. 2014; 385:359–375.
Article21. Short KR, Richard M, Verhagen JH, van Riel D, Schrauwen EJ, van den Brand JM, et al. One health, multiple challenges: the inter-species transmission of influenza A virus. One Health. 2015; 1:1–13.
Article22. Wilson ME. Travel and the emergence of infectious diseases. Emerg Infect Dis. 1995; 1:39–46.
Article23. Wilson ME. The traveller and emerging infections: sentinel, courier, transmitter. J Appl Microbiol. 2003; 94:1S–11S.
Article24. Hwang GM, Mahoney PJ, James JH, Lin GC, Berro AD, Keybl MA, et al. A model-based tool to predict the propagation of infectious disease via airports. Travel Med Infect Dis. 2012; 10:32–42.
Article25. Cho SY, Kang JM, Ha YE, Park GE, Lee JY, Ko JH, et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016; 388:994–1001.
Article26. Park WB, Perera RA, Choe PG, Lau EH, Choi SJ, Chun JY, et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015; 21:2186–2189.
Article27. Beugnet F, Chalvet-Monfray K. Impact of climate change in the epidemiology of vector-borne diseases in domestic carnivores. Comp Immunol Microbiol Infect Dis. 2013; 36:559–566.
Article28. Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, et al. An entomological review of invasive mosquitoes in Europe. Bull Entomol Res. 2015; 105:637–663.
Article29. Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013; 5:299–309.30. Stone BL, Tourand Y, Brissette CA. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis. 2017; 17:619–629.
Article31. Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015; 370:20140051.32. Choi YK, Pascua PN, Song MS. Swine influenza viruses: an Asian perspective. Curr Top Microbiol Immunol. 2013; 370:147–172.
Article33. Chen R, Holmes EC. Avian influenza virus exhibits rapid evolutionary dynamics. Mol Biol Evol. 2006; 23:2336–2341.
Article34. Su S, Gu M, Liu D, Cui J, Gao GF, Zhou J, et al. Epidemiology, evolution, and pathogenesis of H7N9 Influenza viruses in five epidemic waves since 2013 in China. Trends Microbiol. 2017; 25:713–728.
Article35. Wang X, Jiang H, Wu P, Uyeki TM, Feng L, Lai S, et al. Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013-17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis. 2017; 17:822–832.
Article36. Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014; 14:763–772.
Article37. Zhan J, Wang Q, Cheng J, Hu B, Li J, Zhan F, et al. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin. 2017; 32:51–62.
Article38. Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, et al. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis. 2014; 20:1358–1361.
Article39. Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis. 2014; 209:816–827.
Article40. Shin SY, Cho OH, Bae IG. Bone marrow suppression and hemophagocytic histiocytes are common findings in Korean severe fever with thrombocytopenia syndrome patients. Yonsei Med J. 2016; 57:1286–1289.
Article41. Oh WS, Yoo JR, Kwon KT, Kim HI, Lee SJ, Jun JB, et al. Effect of early plasma exchange on survival in patients with severe fever with thrombocytopenia syndrome: a multicenter study. Yonsei Med J. 2017; 58:867–871.
Article42. Gai ZT, Zhang Y, Liang MF, Jin C, Zhang S, Zhu CB, et al. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis. 2012; 206:1095–1102.
Article43. Kim WY, Choi W, Park SW, Wang EB, Lee WJ, Jee Y, et al. Nosocomial transmission of severe fever with thrombocytopenia syndrome in Korea. Clin Infect Dis. 2015; 60:1681–1683.
Article44. Bao CJ, Guo XL, Qi X, Hu JL, Zhou MH, Varma JK, et al. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin Infect Dis. 2011; 53:1208–1214.
Article45. Tang X, Wu W, Wang H, Du Y, Liu L, Kang K, et al. Human-to-human transmission of severe fever with thrombocytopenia syndrome bunyavirus through contact with infectious blood. J Infect Dis. 2013; 207:736–739.
Article46. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012; 367:1814–1820.
Article47. Azhar EI, Lanini S, Ippolito G, Zumla A. The middle east respiratory syndrome coronavirus-a continuing risk to global health security. Adv Exp Med Biol. 2017; 972:49–60.48. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016; 14:523–534.
Article49. Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013; 19:1819–1823.
Article50. Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21(st) century. Trop Med Health. 2011; 39:4 Suppl. 3–11.
Article51. Diamond MS, Pierson TC. Molecular insight into dengue virus pathogenesis and its implications for disease control. Cell. 2015; 162:488–492.
Article52. Halstead SB. Dengue. Lancet. 2007; 370:1644–1652.
Article53. Park JH, Lee DW. Dengue fever in South Korea, 2006-2010. Emerg Infect Dis. 2012; 18:1525–1527.
Article54. Miki S, Lee WC, Lee MJ. A comparative study of the trends of imported Dengue cases in Korea and Japan 2011-2015. J Clin Med Res. 2017; 9:650–653.
Article55. Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev. 2016; 29:487–524.
Article56. Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016; 374:951–958.
Article57. Lim SK, Lim JK, Yoon IK. An update on Zika virus in Asia. Infect Chemother. 2017; 49:91–100.
Article58. Jang HC, Park WB, Kim UJ, Chun JY, Choi SJ, Choe PG, et al. First imported case of Zika virus infection into Korea. J Korean Med Sci. 2016; 31:1173–1177.
Article59. Aderem A, Adkins JN, Ansong C, Galagan J, Kaiser S, Korth MJ, et al. A systems biology approach to infectious disease research: innovating the pathogen-host research paradigm. MBio. 2011; 2:e00325–e00310.
Article60. Mei B, Ding X, Xu HZ, Wang MT. Global gene expression changes in human peripheral blood after H7N9 infection. Gene. 2014; 551:255–260.
Article61. Wang Y, Lupiani B, Reddy SM, Lamont SJ, Zhou H. RNA-seq analysis revealed novel genes and signaling pathway associated with disease resistance to avian influenza virus infection in chickens. Poult Sci. 2014; 93:485–493.
Article62. Ertl R, Klein D. Transcriptional profiling of the host cell response to feline immunodeficiency virus infection. Virol J. 2014; 11:52.
Article63. Jones M, Dry IR, Frampton D, Singh M, Kanda RK, Yee MB, et al. RNA-seq analysis of host and viral gene expression highlights interaction between varicella zoster virus and keratinocyte differentiation. PLoS Pathog. 2014; 10:e1003896.
Article64. Rossetto CC, Tarrant-Elorza M, Verma S, Purushothaman P, Pari GS. Regulation of viral and cellular gene expression by Kaposi’s sarcoma-associated herpesvirus polyadenylated nuclear RNA. J Virol. 2013; 87:5540–5553.
Article65. Juranic Lisnic V, Babic Cac M, Lisnic B, Trsan T, Mefferd A, Das Mukhopadhyay C, et al. Dual analysis of the murine cytomegalovirus and host cell transcriptomes reveal new aspects of the virus-host cell interface. PLoS Pathog. 2013; 9:e1003611.
Article66. Park SJ, Kumar M, Kwon HI, Seong RK, Han K, Song JM, et al. Dynamic changes in host gene expression associated with H5N8 avian influenza virus infection in mice. Sci Rep. 2015; 5:16512.
Article67. Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell. 2016; 18:591–596.
Article68. Onorati M, Li Z, Liu F, Sousa AMM, Nakagawa N, Li M, et al. Zika virus disrupts Phospho-TBK1 localization and mitosis in human neuroepithelial stem cells and radial glia. Cell Rep. 2016; 16:2576–2592.
Article69. Ahmed N, Gottschalk S. How to design effective vaccines: lessons from an old success story. Expert Rev Vaccines. 2009; 8:543–546.
Article70. Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009; 10:116–125.
Article71. Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011; 12:786–795.
Article72. Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014; 41:478–492.
Article73. Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS, et al. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science. 2014; 343:313–317.
Article74. Nakaya HI, Hagan T, Duraisingham SS, Lee EK, Kwissa M, Rouphael N, et al. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity. 2015; 43:1186–1198.
Article75. Nakaya HI, Clutterbuck E, Kazmin D, Wang L, Cortese M, Bosinger SE, et al. Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc Natl Acad Sci U S A. 2016; 113:1853–1858.
Article76. Kazmin D, Nakaya HI, Lee EK, Johnson MJ, van der Most R, van den Berg RA, et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A. 2017; 114:2425–2430.
Article77. Li S, Sullivan NL, Rouphael N, Yu T, Banton S, Maddur MS, et al. Metabolic phenotypes of response to vaccination in humans. Cell. 2017; 169:862–877.e17.
Article78. Hoek KL, Samir P, Howard LM, Niu X, Prasad N, Galassie A, et al. A cell-based systems biology assessment of human blood to monitor immune responses after influenza vaccination. PLoS One. 2015; 10:e0118528.
Article79. Mizukami T, Momose H, Kuramitsu M, Takizawa K, Araki K, Furuhata K, et al. System vaccinology for the evaluation of influenza vaccine safety by multiplex gene detection of novel biomarkers in a preclinical study and batch release test. PLoS One. 2014; 9:e101835.
Article80. Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Niño D, et al. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis. 2011; 203:921–929.
Article81. Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009; 9:741–747.
Article82. Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008; 205:3119–3131.
Article83. O'Hagan DT, Friedland LR, Hanon E, Didierlaurent AM. Towards an evidence based approach for the development of adjuvanted vaccines. Curr Opin Immunol. 2017; 47:93–102.84. Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011; 365:1406–1416.
Article85. Vesikari T, Forstên A, Herbinger KH, Cioppa GD, Beygo J, Borkowski A, et al. Safety and immunogenicity of an MF59(®)-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adults and the elderly. Vaccine. 2012; 30:1388–1396.
Article86. Six A, Bellier B, Thomas-Vaslin V, Klatzmann D. Systems biology in vaccine design. Microb Biotechnol. 2012; 5:295–304.
Article87. Henn AD, Wu S, Qiu X, Ruda M, Stover M, Yang H, et al. High-resolution temporal response patterns to influenza vaccine reveal a distinct human plasma cell gene signature. Sci Rep. 2013; 3:2327.
Article88. Chaussabel D, Pascual V, Banchereau J. Assessing the human immune system through blood transcriptomics. BMC Biol. 2010; 8:84.
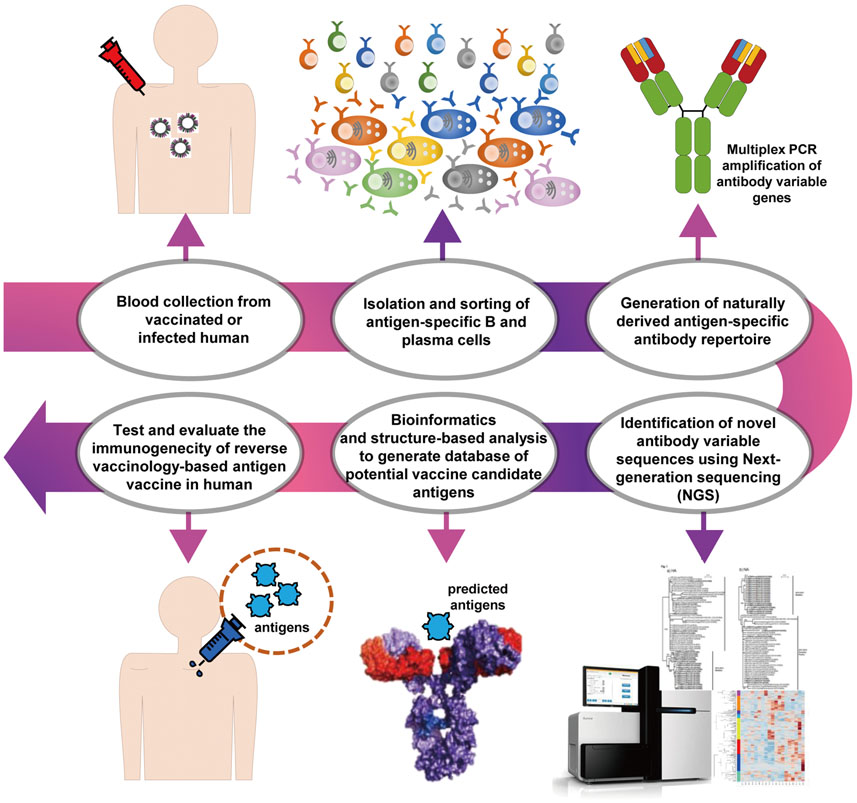
Article89. Rappuoli R. Reverse vaccinology. Curr Opin Microbiol. 2000; 3:445–450.
Article90. Rappuoli R, Black S, Lambert PH. Vaccine discovery and translation of new vaccine technology. Lancet. 2011; 378:360–368.
Article91. Korber B, LaBute M, Yusim K. Immunoinformatics comes of age. PLoS Comput Biol. 2006; 2:e71.
Article92. Sievers SA, Scharf L, West AP Jr, Bjorkman PJ. Antibody engineering for increased potency, breadth and half-life. Curr Opin HIV AIDS. 2015; 10:151–159.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Emerging Infectious Disease due to Micorbial Adaptation: Emergence and Spread of Antimicrobial Resistance
- New Vaccine Technology for Control of Emerging and Reemerging Infectious Diseases
- Systems Bioinformatics Research Trends
- Post COVID-19 Emerging Infectious Diseases: What is the Next Pandemic Agent?
- Suggestions for Advancing the Control of Emerging Infectious Diseases